Page 30 - Low Temperature Energy Systems with Applications of Renewable Energy
P. 30
Principles and operation of refrigeration and heat pump systems 19
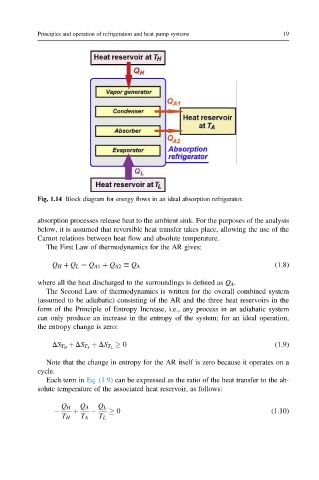
Fig. 1.14 Block diagram for energy flows in an ideal absorption refrigerator.
absorption processes release heat to the ambient sink. For the purposes of the analysis
below, it is assumed that reversible heat transfer takes place, allowing the use of the
Carnot relations between heat flow and absolute temperature.
The First Law of thermodynamics for the AR gives:
Q H þ Q L ¼ Q A1 þ Q A2 h Q A (1.8)
where all the heat discharged to the surroundings is defined as Q A .
The Second Law of thermodynamics is written for the overall combined system
(assumed to be adiabatic) consisting of the AR and the three heat reservoirs in the
form of the Principle of Entropy Increase, i.e., any process in an adiabatic system
can only produce an increase in the entropy of the system; for an ideal operation,
the entropy change is zero:
0 (1.9)
DS T H þ DS T A þ DS T L
Note that the change in entropy for the AR itself is zero because it operates on a
cycle.
Each term in Eq. (1.9) can be expressed as the ratio of the heat transfer to the ab-
solute temperature of the associated heat reservoir, as follows:
Q H Q A Q L
þ 0 (1.10)
T H T A T L