Page 161 - Macromolecular Crystallography
P. 161
150 MACROMOLECULAR CRYS TALLOGRAPHY
(a) (b)
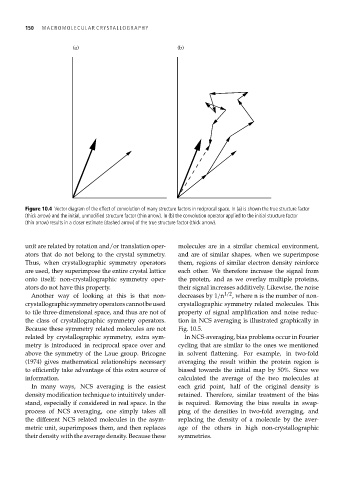
Figure 10.4 Vector diagram of the effect of convolution of many structure factors in reciprocal space. In (a) is shown the true structure factor
(thick arrow) and the initial, unmodified structure factor (thin arrow). In (b) the convolution operator applied to the initial structure factor
(thin arrow) results in a closer estimate (dashed arrow) of the true structure factor (thick arrow).
unit are related by rotation and/or translation oper- molecules are in a similar chemical environment,
ators that do not belong to the crystal symmetry. and are of similar shapes, when we superimpose
Thus, when crystallographic symmetry operators them, regions of similar electron density reinforce
are used, they superimpose the entire crystal lattice each other. We therefore increase the signal from
onto itself; non-crystallographic symmetry oper- the protein, and as we overlay multiple proteins,
ators do not have this property. their signal increases additively. Likewise, the noise
Another way of looking at this is that non- decreases by 1/n 1/2 , where n is the number of non-
crystallographicsymmetryoperatorscannotbeused crystallographic symmetry related molecules. This
to tile three-dimensional space, and thus are not of property of signal amplification and noise reduc-
the class of crystallographic symmetry operators. tion in NCS averaging is illustrated graphically in
Because these symmetry related molecules are not Fig. 10.5.
related by crystallographic symmetry, extra sym- In NCS-averaging, bias problems occur in Fourier
metry is introduced in reciprocal space over and cycling that are similar to the ones we mentioned
above the symmetry of the Laue group. Bricogne in solvent flattening. For example, in two-fold
(1974) gives mathematical relationships necessary averaging the result within the protein region is
to efficiently take advantage of this extra source of biased towards the initial map by 50%. Since we
information. calculated the average of the two molecules at
In many ways, NCS averaging is the easiest each grid point, half of the original density is
density modification technique to intuitively under- retained. Therefore, similar treatment of the bias
stand, especially if considered in real space. In the is required. Removing the bias results in swap-
process of NCS averaging, one simply takes all ping of the densities in two-fold averaging, and
the different NCS related molecules in the asym- replacing the density of a molecule by the aver-
metric unit, superimposes them, and then replaces age of the others in high non-crystallographic
their density with the average density. Because these symmetries.