Page 162 - Macromolecular Crystallography
P. 162
PHASE REFINEMENT THROUGH DENSITY MODIFICATION 151
(a) of building blocks leads to interesting similarities
between the electron density across protein folds
and families. These similarities can be exploited,
and can provide a path to improve poor electron
density.
Histogram matching is a technique that took its
cue from the field of image processing. In various
fields of study, one common problem is that of con-
trolling contrast and brightness of an image. One
(b)
such field is map making using aerial photography.
Due to the wide variety of conditions and ground
types, the aerial photographs are often badly cor-
rected for contrast and brightness. One way of fixing
this problem is that of histogram matching. In his-
togram matching, one does not look at the image
per se, but instead, the histogram of the intensity
values of each pixel, binning them into their appro-
(c) 1
priate histogram area. After making a histogram of
your model, comparing it to a histogram of a known
good image and changing the model so that its his-
togram resembles the known image, the contrast
and brightness of the starting image are dramatically
improved.
As was mentioned before, solvent flattening helps
to improve the low contrast solvent region, whereas
(d) histogram matching helps to improve the high con-
trast protein region. In addition, histogram match-
ing may be used for phase extension, where by
adding and phasing thin shells of data in recipro-
cal space, good phase estimates for structure factors
of previously unphased structure factors can be
obtained. One situation where this happens often
is when solving a structure by MIR. Often, the
diffraction pattern of a protein modified by isomor-
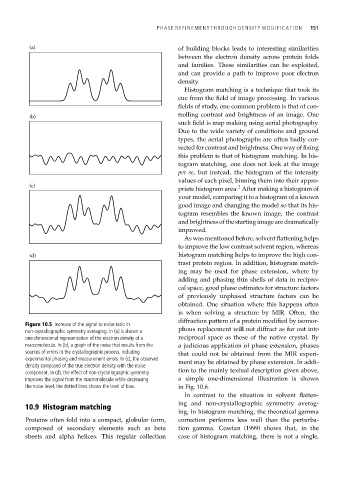
Figure 10.5 Increase of the signal to noise ratio in
non-crystallographic symmetry averaging. In (a) is shown a phous replacement will not diffract as far out into
one-dimensional representation of the electron density of a reciprocal space as those of the native crystal. By
macromolecule. In (b), a graph of the noise that results from the a judicious application of phase extension, phases
sources of errors in the crystallographic process, including that could not be obtained from the MIR experi-
experimental phasing and measurement errors. In (c), the observed ment may be obtained by phase extension. In addi-
density composed of the true electron density with the noise
component. In (d), the effect of non-crystallographic symmetry tion to the mainly textual description given above,
improves the signal from the macromolecule while decreasing a simple one-dimensional illustration is shown
the noise level, the dotted lines shows the level of bias. in Fig. 10.6.
In contrast to the situation in solvent flatten-
ing and non-crystallographic symmetry averag-
10.9 Histogram matching
ing, in histogram matching, the theoretical gamma
Proteins often fold into a compact, globular form, correction performs less well than the perturba-
composed of secondary elements such as beta tion gamma. Cowtan (1999) shows that, in the
sheets and alpha helices. This regular collection case of histogram matching, there is not a single,