Page 136 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 136
Section 4.8 Hardenability of Ferrous Alloys
400 400 ’
Ferrite +
5 Ferrite + pearlite / 5 fine pearlite
EE 13,
3 n*
9)
Q, 200 Q, 200
C C
E 'E
Fefflie +
I cu Spheroldite I co coarse pearlite
0 0
0 0.2 0.4 0.6 0.8 1.0 %C O 0.2 0.4 0.6 0.8 1.0 %C
0 3 6 9 12 15 % Fe3C 0 3 6 9 12 15 %Fe3C
0 25 50 75 100 97 %Pea|'lile 0 25 50 75 100 97 % Pearlite
(H) (D)
A Spheroidite
3 100
(I)
(D
cv
E
§ 50 Ferrite +
I- pearlite
0 _
O 0.2 0.4 0.6 0.8 1.0%C
0 3 6 9 12 15 %Fe3C
O 25 50 75 100 97 % Pearlite
(C)
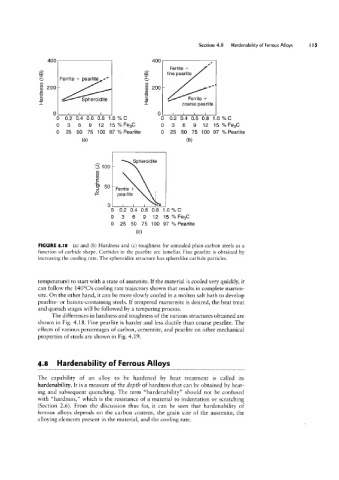
FIGURE 4.l8 (a) and lb) Hardness and (c) toughness for annealed plain-carbon steels as a
function of carbide shape. Carbides in the pearlite are lamellar. Fine pearlite is obtained by
increasing the cooling rate. The spheroidite structure has spherelike carbide particles.
temperature) to start with a state of austenite. If the material is cooled very quickly, it
can follow the 140°C/s cooling rate trajectory shown that results in complete marten-
site. Un the other hand, it can be more slowly cooled in a molten salt bath to develop
pearlite- or bainite-containing steels. If tempered martensite is desired, the heat treat
and quench stages will be followed by a tempering process.
The differences in hardness and toughness of the various structures obtained are
shown in Fig. 4.18. Fine pearlite is harder and less ductile than coarse pearlite. The
effects of various percentages of carbon, cementite, and pearlite on other mechanical
properties of steels are shown in Fig. 4.19.
4.8 Hardenability of Ferrous Alloys
The capability of an alloy to be hardened by heat treatment is called its
hardenability. It is a measure of the dept/0 of hardness that can be obtained by heat-
ing and subsequent quenching. The term “hardenability” should not be confused
with “hardness,” which is the resistance of a material to indentation or scratching
(Section 2.6). From the discussion thus far, it can be seen that hardenability of
ferrous alloys depends on the carbon content, the grain size of the austenite, the
alloying elements present in the material, and the cooling rate.