Page 138 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 138
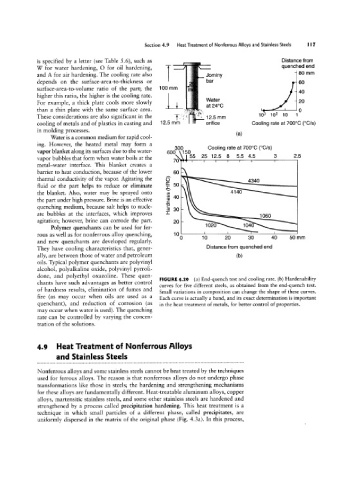
W for water hardening, O for oil hardening, Distance from
I I7
Section 4.9
Heat Treatment of Nonferrous Alloys and Stainless Steels
is specified by a letter (see Table 5 .6), such as
80 mm
and A for air hardening. The cooling rate also Jominy flUenCh9d end
depends on the surface-area-to-thickness or 100 mm bar 60
surface-area-to-volume ratio of the part; the 40
higher this ratio, the higher is the cooling rate.
For example, a thick plate cools more slowly l/Yiifc 20
than a thin plate with the same surface area. N a 0
These considerations are also significant in the '05 125 mm 103 102 10 1
cooling of metals and of plastics in casting and 12-5 mm orifice Cooling rate at 700°C (°C/S)
in molding processes.
Water is a common medium for rapid cool- (a)
ing. However, the heated metal may form a 0 O
vapor blanket along its surfaces due to the water- 500300 50 Cooling rate at 700 C ( C/S)
vapor bubbles that form when water boils at the 70 05 05 1?-5 0 5;5 4;5 0 2-5
metal-water interface. This blanket creates a
barrier to heat conduction, because of the lower 60
thermal conductivity of the vapor. Agitating the G 4340
fluid or the part helps to reduce or eliminate § 50 e
the blanket. Also, water may be sprayed onto T5 4140
the part under high pressure. Brine is an effective 0 40 ' `
C
quenching medium, because salt helps to nucle- E 30 _
ate bubbles at the interfaces, which improves I 1060
agitation; however, brine can corrode the part. 20 _ TT;
Polymer quenchants can be used for fer- 1020 1040 T
rous as well as for nonferrous alloy quenching, 100 1‘O 2'O 30 1 4'O 50 :nm
and new quenchants are developed regularly.
They have cooling characteristics that, gener- DlS1af1C9 ffom quenched 9110
ally, are between those of water and petroleum (b)
oils. Typical polymer quenchants are polyvinyl
alcohol, polyalkaline oxide, polyvinyl pyrroli-
done, and polyethyl oxazoline. These quen- FIGURE 4.20 (a) End-quench test and cooling rate. (b) Hardenability
chants have such advantages as better control
curves for five different steels, as obtained from the end-quench test.
of hardness results, elimination of fumes and Small variations in composition can change the shape of these curves.
fire (as may occur when oils are used as a Each curve is actually a band, and its exact determination is important
quenchant), and reduction of corrosion (as in the heat treatment of metals, for better control of properties.
may occur when water is used). The quenching
rate can be controlled by varying the concen-
tration of the solutions.
4.9 Heat Treatment of Nonferrous Alloys
and Stainless Steels
Nonferrous alloys and some stainless steels cannot be heat treated by the techniques
used for ferrous alloys. The reason is that nonferrous alloys do not undergo phase
transformations like those in steels; the hardening and strengthening mechanisms
for these alloys are fundamentally different. Heat-treatable aluminum alloys, copper
alloys, martensitic stainless steels, and some other stainless steels are hardened and
strengthened by a process called precipitation hardening. This heat treatment is a
technique in which small particles of a different phase, called precipitates, are
uniformly dispersed in the matrix of the original phase (Fig. 4.3a). In this process,