Page 132 - Materials Science and Engineering An Introduction
P. 132
104 • Chapter 3 / The Structure of Crystalline Solids
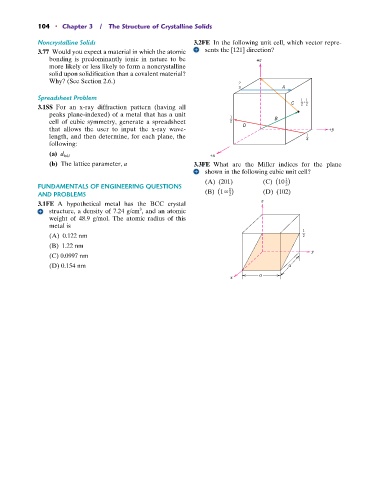
Noncrystalline Solids 3.2FE In the following unit cell, which vector repre-
3.77 Would you expect a material in which the atomic sents the [121] direction?
bonding is predominantly ionic in nature to be +z
more likely or less likely to form a noncrystalline
solid upon solidification than a covalent material?
Why? (See Section 2.6.) 2
3 A
Spreadsheet Problem 1 , 1
C 2 2
3.1SS For an x-ray diffraction pattern (having all
peaks plane-indexed) of a metal that has a unit 1
cell of cubic symmetry, generate a spreadsheet 2 B
that allows the user to input the x-ray wave- D +y
length, and then determine, for each plane, the 1
following: 4
(a) d hkl +x
(b) The lattice parameter, a 3.3FE What are the Miller indices for the plane
shown in the following cubic unit cell?
1
(A) (201) (C) (10 )
FUNDAMENTALS OF ENGINEERING QUESTIONS 1 2
AND PROBLEMS (B) (1 ) (D) (102)
2
3.1FE A hypothetical metal has the BCC crystal z
3
structure, a density of 7.24 g/cm , and an atomic
weight of 48.9 g/mol. The atomic radius of this
metal is
1
(A) 0.122 nm 2
(B) 1.22 nm
y
(C) 0.0997 nm
(D) 0.154 nm a
a
x