Page 131 - Materials Science and Engineering An Introduction
P. 131
Questions and Problems • 103
a wavelength of 0.1659 nm is used, compute the (a) Determine the interplanar spacing for each of
following: the peaks.
(a) The interplanar spacing for this set of planes (b) For each peak, determine the atomic radius
(b) The atomic radius for the Nb atom for Pt, and compare these with the value pre-
sented in Table 3.1.
3.71 For which set of crystallographic planes will a first-
order diffraction peak occur at a diffraction angle 3.75 The following table lists diffraction angles for the
of 44.53 for FCC nickel (Ni) when monochromatic first three peaks (first-order) of the x-ray diffrac-
radiation having a wavelength of 0.1542 nm is used? tion pattern for some metal. Monochromatic x-ra-
diation having a wavelength of 0.1397 nm was used.
3.72 For which set of crystallographic planes will a
first-order diffraction peak occur at a diffraction (a) Determine whether this metal’s crystal struc-
angle of 44.53 for BCC tantalum (Ta) when ture is FCC, BCC, or neither FCC or BCC, and
monochromatic radiation having a wavelength of explain the reason for your choice.
0.1937 nm is used? (b) If the crystal structure is either BCC or FCC,
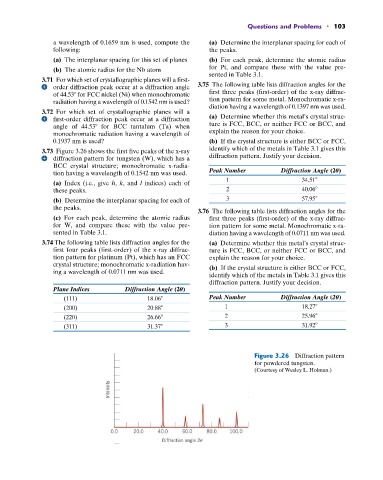
3.73 Figure 3.26 shows the first five peaks of the x-ray identify which of the metals in Table 3.1 gives this
diffraction pattern for tungsten (W), which has a diffraction pattern. Justify your decision.
BCC crystal structure; monochromatic x-radia-
tion having a wavelength of 0.1542 nm was used. Peak Number Diffraction Angle (2U)
1 34.51
(a) Index (i.e., give h, k, and l indices) each of
these peaks. 2 40.06
(b) Determine the interplanar spacing for each of 3 57.95
the peaks.
3.76 The following table lists diffraction angles for the
(c) For each peak, determine the atomic radius first three peaks (first-order) of the x-ray diffrac-
for W, and compare these with the value pre- tion pattern for some metal. Monochromatic x-ra-
sented in Table 3.1. diation having a wavelength of 0.0711 nm was used.
3.74 The following table lists diffraction angles for the (a) Determine whether this metal’s crystal struc-
first four peaks (first-order) of the x-ray diffrac- ture is FCC, BCC, or neither FCC or BCC, and
tion pattern for platinum (Pt), which has an FCC explain the reason for your choice.
crystal structure; monochromatic x-radiation hav- (b) If the crystal structure is either BCC or FCC,
ing a wavelength of 0.0711 nm was used.
identify which of the metals in Table 3.1 gives this
diffraction pattern. Justify your decision.
Plane Indices Diffraction Angle (2U)
(111) 18.06 Peak Number Diffraction Angle (2U)
(200) 20.88 1 18.27
(220) 26.66 2 25.96
(311) 31.37 3 31.92
Figure 3.26 Diffraction pattern
for powdered tungsten.
(Courtesy of Wesley L. Holman.)