Page 126 - Materials Science and Engineering An Introduction
P. 126
98 • Chapter 3 / The Structure of Crystalline Solids
Density Computations
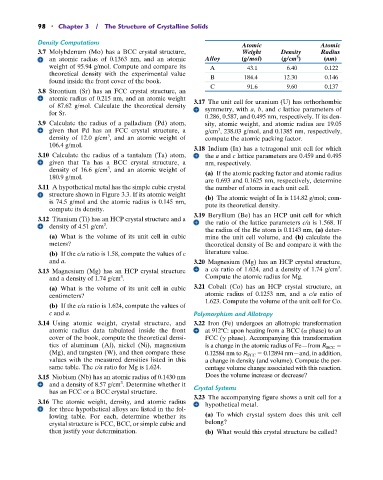
Atomic Atomic
3.7 Molybdenum (Mo) has a BCC crystal structure, Weight Density Radius
3
an atomic radius of 0.1363 nm, and an atomic Alloy (g/mol) (g/cm ) (nm)
weight of 95.94 g/mol. Compute and compare its A 43.1 6.40 0.122
theoretical density with the experimental value
found inside the front cover of the book. B 184.4 12.30 0.146
C 91.6 9.60 0.137
3.8 Strontium (Sr) has an FCC crystal structure, an
atomic radius of 0.215 nm, and an atomic weight 3.17 The unit cell for uranium (U) has orthorhombic
of 87.62 g/mol. Calculate the theoretical density lattice parameters of
for Sr. symmetry, with a, b, and c
0.286, 0.587, and 0.495 nm, respectively. If its den-
3.9 Calculate the radius of a palladium (Pd) atom, sity, atomic weight, and atomic radius are 19.05
3
given that Pd has an FCC crystal structure, a g/cm , 238.03 g/mol, and 0.1385 nm, respectively,
3
density of 12.0 g/cm , and an atomic weight of compute the atomic packing factor.
106.4 g/mol.
3.18 Indium (In) has a tetragonal unit cell for which
3.10 Calculate the radius of a tantalum (Ta) atom, the a and c lattice parameters are 0.459 and 0.495
given that Ta has a BCC crystal structure, a nm, respectively.
3
density of 16.6 g/cm , and an atomic weight of (a) If the atomic packing factor and atomic radius
180.9 g/mol.
are 0.693 and 0.1625 nm, respectively, determine
3.11 A hypothetical metal has the simple cubic crystal the number of atoms in each unit cell.
structure shown in Figure 3.3. If its atomic weight (b) The atomic weight of In is 114.82 g/mol; com-
is 74.5 g/mol and the atomic radius is 0.145 nm, pute its theoretical density.
compute its density.
3.19 Beryllium (Be) has an HCP unit cell for which
3.12 Titanium (Ti) has an HCP crystal structure and a the ratio of the lattice parameters c/a is 1.568. If
3
density of 4.51 g/cm .
the radius of the Be atom is 0.1143 nm, (a) deter-
(a) What is the volume of its unit cell in cubic mine the unit cell volume, and (b) calculate the
meters? theoretical density of Be and compare it with the
(b) If the c/a ratio is 1.58, compute the values of c literature value.
and a. 3.20 Magnesium (Mg) has an HCP crystal structure,
3
3.13 Magnesium (Mg) has an HCP crystal structure a c/a ratio of 1.624, and a density of 1.74 g/cm .
3
and a density of 1.74 g/cm . Compute the atomic radius for Mg.
(a) What is the volume of its unit cell in cubic 3.21 Cobalt (Co) has an HCP crystal structure, an
centimeters? atomic radius of 0.1253 nm, and a c/a ratio of
1.623. Compute the volume of the unit cell for Co.
(b) If the c/a ratio is 1.624, compute the values of
c and a. Polymorphism and Allotropy
3.14 Using atomic weight, crystal structure, and 3.22 Iron (Fe) undergoes an allotropic transformation
atomic radius data tabulated inside the front at 912 C: upon heating from a BCC (a phase) to an
cover of the book, compute the theoretical densi- FCC (g phase). Accompanying this transformation
ties of aluminum (Al), nickel (Ni), magnesium is a change in the atomic radius of Fe—from R BCC
(Mg), and tungsten (W), and then compare these 0.12584 nm to R FCC 0.12894 nm—and, in addition,
values with the measured densities listed in this a change in density (and volume). Compute the per-
same table. The c/a ratio for Mg is 1.624. centage volume change associated with this reaction.
3.15 Niobium (Nb) has an atomic radius of 0.1430 nm Does the volume increase or decrease?
3
and a density of 8.57 g/cm . Determine whether it
has an FCC or a BCC crystal structure. Crystal Systems
3.23 The accompanying figure shows a unit cell for a
3.16 The atomic weight, density, and atomic radius hypothetical metal.
for three hypothetical alloys are listed in the fol-
lowing table. For each, determine whether its (a) To which crystal system does this unit cell
crystal structure is FCC, BCC, or simple cubic and belong?
then justify your determination. (b) What would this crystal structure be called?