Page 129 - Materials Science and Engineering An Introduction
P. 129
Questions and Problems • 101
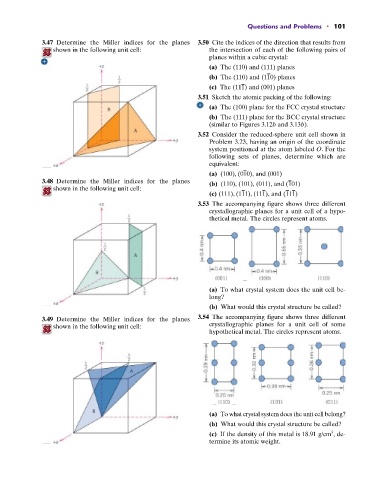
3.47 Determine the Miller indices for the planes 3.50 Cite the indices of the direction that results from
shown in the following unit cell: the intersection of each of the following pairs of
planes within a cubic crystal:
(a) The (110) and (111) planes
(b) The (110) and (110) planes
(c) The (111) and (001) planes
3.51 Sketch the atomic packing of the following:
(a) The (100) plane for the FCC crystal structure
(b) The (111) plane for the BCC crystal structure
(similar to Figures 3.12b and 3.13b).
3.52 Consider the reduced-sphere unit cell shown in
Problem 3.23, having an origin of the coordinate
system positioned at the atom labeled O. For the
following sets of planes, determine which are
equivalent:
(a) (100), (010), and (001)
3.48 Determine the Miller indices for the planes (b) (110), (101), (011), and (101)
shown in the following unit cell:
(c) (111), (111), (111), and (111)
3.53 The accompanying figure shows three different
crystallographic planes for a unit cell of a hypo-
thetical metal. The circles represent atoms.
(a) To what crystal system does the unit cell be-
long?
(b) What would this crystal structure be called?
3.49 Determine the Miller indices for the planes 3.54 The accompanying figure shows three different
shown in the following unit cell: crystallographic planes for a unit cell of some
hypothetical metal. The circles represent atoms.
(a) To what crystal system does the unit cell belong?
(b) What would this crystal structure be called?
3
(c) If the density of this metal is 18.91 g/cm , de-
termine its atomic weight.