Page 127 - Materials Science and Engineering An Introduction
P. 127
Questions and Problems • 99
(c) Calculate the density of the material, given Crystallographic Directions
that its atomic weight is 141 g/mol. 3.31 Draw an orthorhombic unit cell, and within that
+z cell, a [2 1 1] direction.
3.32 Sketch a monoclinic unit cell, and within that
cell, a [1 0 1] direction.
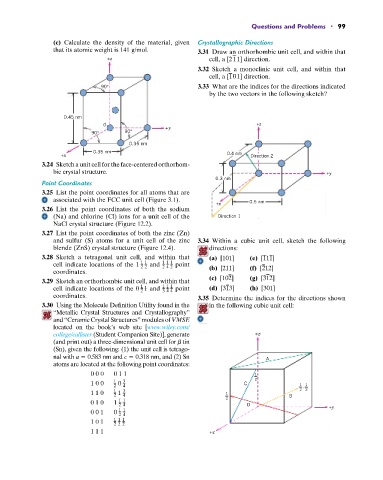
90° 3.33 What are the indices for the directions indicated
by the two vectors in the following sketch?
0.45 nm
O +z
+y
90° 90°
0.35 nm
0.35 nm
+x 0.4 nm Direction 2
3.24 Sketch a unit cell for the face-centered orthorhom-
bic crystal structure. +y
0.3 nm
Point Coordinates
3.25 List the point coordinates for all atoms that are
associated with the FCC unit cell (Figure 3.1). 0.5 nm
+x
3.26 List the point coordinates of both the sodium
(Na) and chlorine (Cl) ions for a unit cell of the Direction 1
NaCl crystal structure (Figure 12.2).
3.27 List the point coordinates of both the zinc (Zn)
and sulfur (S) atoms for a unit cell of the zinc 3.34 Within a cubic unit cell, sketch the following
blende (ZnS) crystal structure (Figure 12.4). directions:
3.28 Sketch a tetragonal unit cell, and within that (a) [101] (e) [111]
1 1
1 1 1
cell indicate locations of the 1 and point [211]
2 2
2 4 2
coordinates. (b) (f) [212]
3.29 Sketch an orthorhombic unit cell, and within that (c) [102] (g) [312]
1 1 1
1
cell indicate locations of the 0 1 and point (d) [313] (h) [301]
3 4 4
2
coordinates. 3.35 Determine the indices for the directions shown
3.30 Using the Molecule Definition Utility found in the in the following cubic unit cell:
“Metallic Crystal Structures and Crystallography”
and “Ceramic Crystal Structures” modules of VMSE
located on the book’s web site [www.wiley.com/
college/callister (Student Companion Site)], generate +z
(and print out) a three-dimensional unit cell for b tin
(Sn), given the following: (1) the unit cell is tetrago-
nal with a 0.583 nm and c 0.318 nm, and (2) Sn A
atoms are located at the following point coordinates:
0 0 0 0 1 1 1
1 0 0 1 0 3 C 2
2 4 1 1
,
1 1 0 1 1 3 1 2 2
2 4 B
0 1 0 1 2 D
1 1
2 4
0 0 1 0 +y
1 1
2 4
1 0 1 1 1 1
2 2 2
1 1 1 +x