Page 121 - Materials Science and Engineering An Introduction
P. 121
Summary • 93
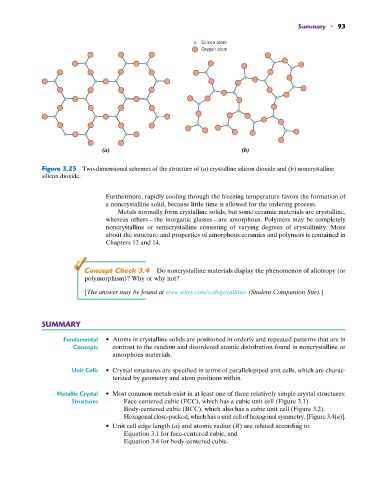
Silicon atom
Oxygen atom
(a) (b)
Figure 3.25 Two-dimensional schemes of the structure of (a) crystalline silicon dioxide and (b) noncrystalline
silicon dioxide.
Furthermore, rapidly cooling through the freezing temperature favors the formation of
a noncrystalline solid, because little time is allowed for the ordering process.
Metals normally form crystalline solids, but some ceramic materials are crystalline,
whereas others—the inorganic glasses—are amorphous. Polymers may be completely
noncrystalline or semicrystalline consisting of varying degrees of crystallinity. More
about the structure and properties of amorphous ceramics and polymers is contained in
Chapters 12 and 14.
Concept Check 3.4 Do noncrystalline materials display the phenomenon of allotropy (or
polymorphism)? Why or why not?
[The answer may be found at www.wiley.com/college/callister (Student Companion Site).]
SUMMARY
Fundamental • Atoms in crystalline solids are positioned in orderly and repeated patterns that are in
Concepts contrast to the random and disordered atomic distribution found in noncrystalline or
amorphous materials.
Unit Cells • Crystal structures are specified in terms of parallelepiped unit cells, which are charac-
terized by geometry and atom positions within.
Metallic Crystal • Most common metals exist in at least one of three relatively simple crystal structures:
Structures Face-centered cubic (FCC), which has a cubic unit cell (Figure 3.1).
Body-centered cubic (BCC), which also has a cubic unit cell (Figure 3.2).
Hexagonal close-packed, which has a unit cell of hexagonal symmetry, [Figure 3.4(a)].
• Unit cell edge length (a) and atomic radius (R) are related according to
Equation 3.1 for face-centered cubic, and
Equation 3.4 for body-centered cubic.