Page 372 - Materials Science and Engineering An Introduction
P. 372
344 • Chapter 9 / Phase Diagrams
0.8
Ni
2400
Ti Mo Si W 2200 0.6 Cr
1200
Eutectoid temperature (°C) 1000 Cr 2000 Eutectoid temperature (°F) Eutectoid composition (wt% C) 0.4 Ti Mo Si W Mn
1800
1600
0.2
800
Mn 1400
1200
600 0 0 2 4 6 8 10 12 14
Ni 1000 Concentration of alloying elements (wt%)
0 2 4 6 8 10 12 14
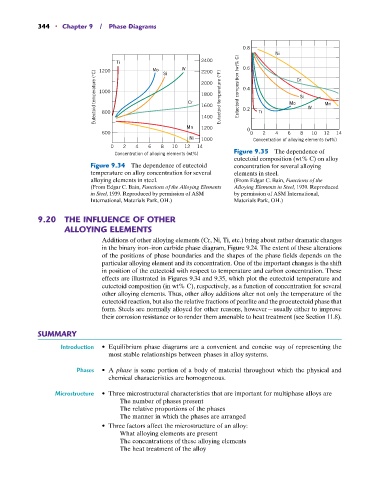
Figure 9.35 The dependence of
Concentration of alloying elements (wt%)
eutectoid composition (wt% C) on alloy
Figure 9.34 The dependence of eutectoid concentration for several alloying
temperature on alloy concentration for several elements in steel.
alloying elements in steel. (From Edgar C. Bain, Functions of the
(From Edgar C. Bain, Functions of the Alloying Elements Alloying Elements in Steel, 1939. Reproduced
in Steel, 1939. Reproduced by permission of ASM by permission of ASM International,
International, Materials Park, OH.) Materials Park, OH.)
9.20 THE INFLUENCE OF OTHER
ALLOYING ELEMENTS
Additions of other alloying elements (Cr, Ni, Ti, etc.) bring about rather dramatic changes
in the binary iron–iron carbide phase diagram, Figure 9.24. The extent of these alterations
of the positions of phase boundaries and the shapes of the phase fields depends on the
particular alloying element and its concentration. One of the important changes is the shift
in position of the eutectoid with respect to temperature and carbon concentration. These
effects are illustrated in Figures 9.34 and 9.35, which plot the eutectoid temperature and
eutectoid composition (in wt% C), respectively, as a function of concentration for several
other alloying elements. Thus, other alloy additions alter not only the temperature of the
eutectoid reaction, but also the relative fractions of pearlite and the proeutectoid phase that
form. Steels are normally alloyed for other reasons, however—usually either to improve
their corrosion resistance or to render them amenable to heat treatment (see Section 11.8).
SUMMARY
Introduction • Equilibrium phase diagrams are a convenient and concise way of representing the
most stable relationships between phases in alloy systems.
Phases • A phase is some portion of a body of material throughout which the physical and
chemical characteristics are homogeneous.
Microstructure • Three microstructural characteristics that are important for multiphase alloys are
The number of phases present
The relative proportions of the phases
The manner in which the phases are arranged
• Three factors affect the microstructure of an alloy:
What alloying elements are present
The concentrations of these alloying elements
The heat treatment of the alloy