Page 117 - Materials Chemistry, Second Edition
P. 117
104 2 Solid-State Chemistry
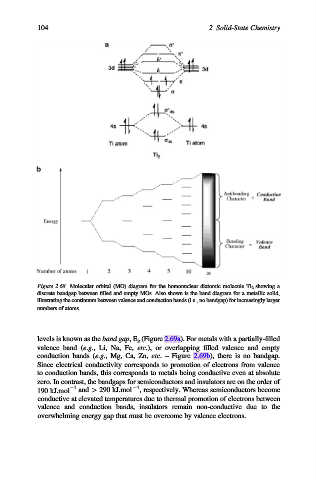
Figure 2.68. Molecular orbital (MO) diagram for the homonuclear diatomic molecule Ti 2 showing a
discrete bandgap between filled and empty MOs. Also shown is the band diagram for a metallic solid,
illustrating the continuum between valence and conduction bands (i.e., no bandgap) for increasingly larger
numbers of atoms.
levels is known as the band gap,E g (Figure 2.69a). For metals with a partially-filled
valence band (e.g., Li, Na, Fe, etc.), or overlapping filled valence and empty
conduction bands (e.g., Mg, Ca, Zn, etc. – Figure 2.69b), there is no bandgap.
Since electrical conductivity corresponds to promotion of electrons from valence
to conduction bands, this corresponds to metals being conductive even at absolute
zero. In contrast, the bandgaps for semiconductors and insulators are on the order of
1
190 kJ.mol 1 and > 290 kJ.mol , respectively. Whereas semiconductors become
conductive at elevated temperatures due to thermal promotion of electrons between
valence and conduction bands, insulators remain non-conductive due to the
overwhelming energy gap that must be overcome by valence electrons.