Page 122 - Materials Chemistry, Second Edition
P. 122
109
2.3. The Crystalline State
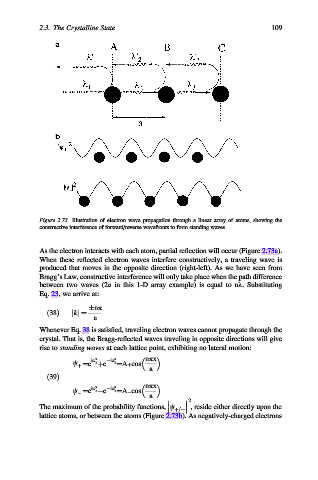
Figure 2.73. Illustration of electron wave propagation through a linear array of atoms, showing the
constructive interference of forward/reverse wavefronts to form standing waves.
As the electron interacts with each atom, partial reflection will occur (Figure 2.73a).
When these reflected electron waves interfere constructively, a traveling wave is
produced that moves in the opposite direction (right-left). As we have seen from
Bragg’s Law, constructive interference will only take place when the path difference
between two waves (2a in this 1-D array example) is equal to nl. Substituting
Eq. 23, we arrive at:
np
ð38Þ k jj ¼
a
Whenever Eq. 38 is satisfied, traveling electron waves cannot propagate through the
crystal. That is, the Bragg-reflected waves traveling in opposite directions will give
rise to standing waves at each lattice point, exhibiting no lateral motion:
npx
x
x
ip
c ¼e þe ip a ¼A þ cos
a
þ
a
ð39Þ
npx
x
x
ip
c ¼e e ip a ¼A cos
a
a
2
, reside either directly upon the
þ=
The maximum of the probability functions, c
lattice atoms, or between the atoms (Figure 2.73b). As negatively-charged electrons