Page 243 - Materials Chemistry, Second Edition
P. 243
230 3 Metals
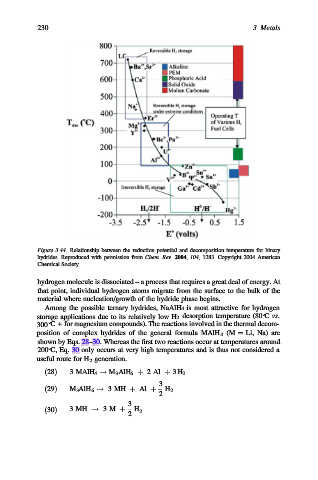
Figure 3.44. Relationship between the reduction potential and decomposition temperature for binary
hydrides. Reproduced with permission from Chem. Rev. 2004, 104, 1283. Copyright 2004 American
Chemical Society.
hydrogen molecule is dissociated – a process that requires a great deal of energy. At
that point, individual hydrogen atoms migrate from the surface to the bulk of the
material where nucleation/growth of the hydride phase begins.
Among the possible ternary hydrides, NaAlH 4 is most attractive for hydrogen
storage applications due to its relatively low H 2 desorption temperature (80 C vs.
300 C + for magnesium compounds). The reactions involved in the thermal decom-
position of complex hydrides of the general formula MAlH 4 (M ¼ Li, Na) are
shown by Eqs. 28–30. Whereas the first two reactions occur at temperatures around
200 C, Eq. 30 only occurs at very high temperatures and is thus not considered a
useful route for H 2 generation.
ð28Þ 3MAlH 4 ! M 3 AlH 6 þ 2Al þ 3H 2
3
ð29Þ M 3 AlH 6 ! 3MH þ Al þ H 2
2
3
ð30Þ 3MH ! 3M þ H 2
2