Page 254 - Materials Chemistry, Second Edition
P. 254
241
4.1. Properties and Types of Semiconductors
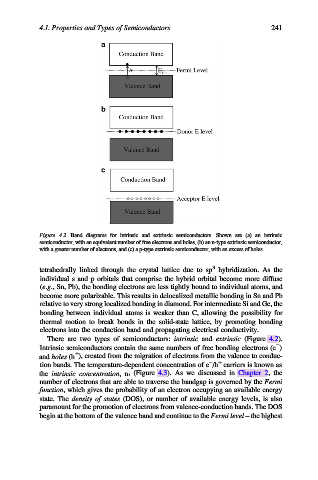
Figure 4.2. Band diagrams for intrinsic and extrinsic semiconductors. Shown are (a) an intrinsic
semiconductor, with an equivalent number of free electrons and holes, (b) an n-type extrinsic semiconductor,
with a greater number of electrons, and (c) a p-type extrinsic semiconductor, with an excess of holes.
3
tetrahedrally linked through the crystal lattice due to sp hybridization. As the
individual s and p orbitals that comprise the hybrid orbital become more diffuse
(e.g., Sn, Pb), the bonding electrons are less tightly bound to individual atoms, and
become more polarizable. This results in delocalized metallic bonding in Sn and Pb
relative to very strong localized bonding in diamond. For intermediate Si and Ge, the
bonding between individual atoms is weaker than C, allowing the possibility for
thermal motion to break bonds in the solid-state lattice, by promoting bonding
electrons into the conduction band and propagating electrical conductivity.
There are two types of semiconductors: intrinsic and extrinsic (Figure 4.2).
Intrinsic semiconductors contain the same numbers of free bonding electrons (e )
+
and holes (h ), created from the migration of electrons from the valence to conduc-
+
tion bands. The temperature-dependent concentration of e /h carriers is known as
the intrinsic concentration,n i (Figure 4.3). As we discussed in Chapter 2, the
number of electrons that are able to traverse the bandgap is governed by the Fermi
function, which gives the probability of an electron occupying an available energy
state. The density of states (DOS), or number of available energy levels, is also
paramount for the promotion of electrons from valence-conduction bands. The DOS
begin at the bottom of the valence band and continue to the Fermi level – the highest