Page 252 - Mechanical Engineer's Data Handbook
P. 252
240 MECHANICAL ENGINEER'S DATA HANDBOOK
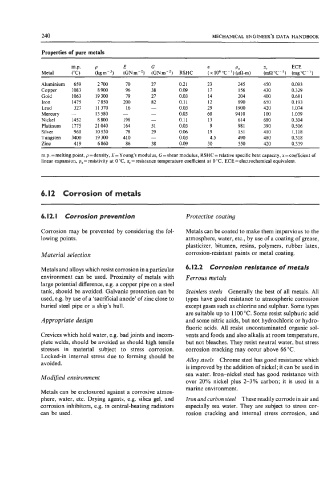
Properties of pure metals
m.p. P E G a PO a, ECE
Metal ("C) (kgm-') (GNm-') (GNm-*) RSHC (x 1060C-')(@-m) (mQ'C-') (mg"C-')
Aluminium 659 2 700 70 27 0.21 23 245 450 0.093
Copper 1083 8900 96 38 0.09 17 156 430 0.329
Gold 1063 19300 19 27 0.03 14 204 400 0.68 1
Iron 1475 7850 200 82 0.11 12 890 650 0.193
Lead 327 11 370 16 - 0.03 29 1900 420 1.074
Mercury - 13580 - - 0.03 60 9410 100 1.039
Nickel 1452 8 800 198 - 0.1 1 13 614 680 0.304
Platinum 1775 21040 164 51 0.03 9 98 1 390 0.506
Silver 961 10530 78 29 0.06 19 151 410 1.118
Tungsten 3400 19300 410 - 0.03 4.5 490 480 0.318
Zinc 419 6 860 86 38 0.09 30 550 420 0.339
m.p. =melting point, p =density, E=Young's modulus, G = shear modulus, RSHC=relative specific heat capacity, a=coefficient of
linear expansion, p, = resistivity at 0 "C, a, = resistance temperature coefficient at 0 "C, ECE = electrochemical equivalent.
6.12 Corrosion of metals
6.12. I Corrosion prevention Protective coating
Corrosion may be prevented by considering the fol- Metals can be coated to make them impervious to the
lowing points. atmosphere, water, etc., by use of a coating of grease,
plasticizer, bitumen, resins, polymers, rubber latex,
Material selection corrosion-resistant paints or metal coating.
Metals and alloys which resist corrosion in a particular 6.12.2 Corrosion resistance of metals
environment can be used. Proximity of metals with Ferrous metals
large potential difference, e.g. a copper pipe on a steel
tank, should be avoided. Galvanic protection can be Stainless steels Generally the best of all metals. All
used, e.g. by use of a 'sacrificial anode' of zinc close to types have good resistance to atmospheric corrosion
buried steel pipe or a ship's hull. except gases such as chlorine and sulphur. Some types
are suitable up to 1100 "C. Some resist sulphuric acid
Appropriate design and some nitric acids, but not hydrochloric or hydro-
fluoric acids. All resist uncontaminated organic sol-
Crevices which hold water, e.g. bad joints and incom- vents and foods and also alkalis at room temperature,
plete welds, should be avoided as should high tensile but not bleaches. They resist neutral water, but stress
stresses in material subject to stress corrosion. corrosion cracking may occur above 66 "C.
Locked-in internal stress due to forming should be
avoided. Alloy steels Chrome steel has good resistance which
is improved by the addition of nickel; it can be used in
Modijied environment sea water. Iron-nickel steel has good resistance with
over 20% nickel plus 2-3% carbon; it is used in a
marine environment.
Metals can be enclosured against a corrosive atmos-
phere, water, etc. Drying agents, e.g. silica gel, and Iron and carbon steel These readily corrode in air and
corrosion inhibitors, e.g. in central-heating radiators especially sea water. They are subject to stress cor-
can be used. rosion cracking and internal stress corrosion, and