Page 205 - Mechanical Engineers' Handbook (Volume 4)
P. 205
194 Heat-Transfer Fundamentals
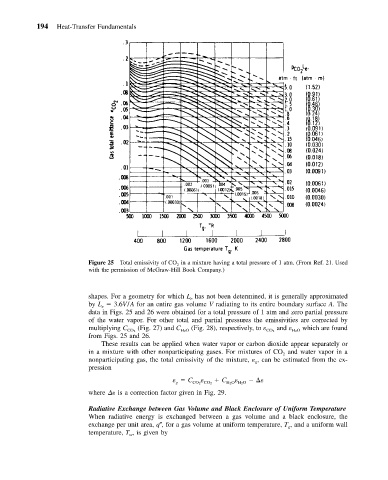
Figure 25 Total emissivity of CO 2 in a mixture having a total pressure of 1 atm. (From Ref. 21. Used
with the permission of McGraw-Hill Book Company.)
shapes. For a geometry for which L has not been determined, it is generally approximated
e
by L 3.6V/A for an entire gas volume V radiating to its entire boundary surface A. The
e
data in Figs. 25 and 26 were obtained for a total pressure of 1 atm and zero partial pressure
of the water vapor. For other total and partial pressures the emissivities are corrected by
multiplying C (Fig. 27) and C (Fig. 28), respectively, to and which are found
2
CO 2 H O CO 2 H O
2
from Figs. 25 and 26.
These results can be applied when water vapor or carbon dioxide appear separately or
in a mixture with other nonparticipating gases. For mixtures of CO and water vapor in a
2
nonparticipating gas, the total emissivity of the mixture, , can be estimated from the ex-
g
pression
C CO 2 CO 2 C HO HO
g
2
2
where is a correction factor given in Fig. 29.
Radiative Exchange between Gas Volume and Black Enclosure of Uniform Temperature
When radiative energy is exchanged between a gas volume and a black enclosure, the
exchange per unit area, q , for a gas volume at uniform temperature, T , and a uniform wall
g
temperature, T ,isgiven by
w