Page 210 - Mechanical Engineers' Handbook (Volume 4)
P. 210
4 Boiling and Condensation Heat Transfer 199
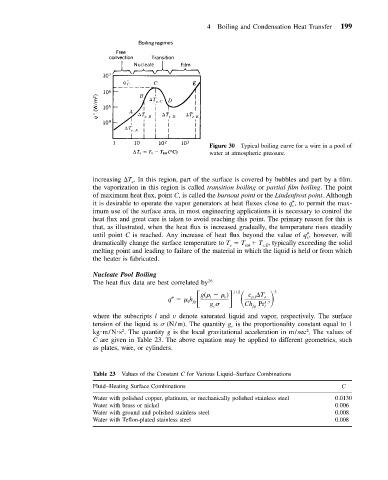
Figure 30 Typical boiling curve for a wire in a pool of
water at atmospheric pressure.
increasing T . In this region, part of the surface is covered by bubbles and part by a film.
e
the vaporization in this region is called transition boiling or partial film boiling. The point
of maximum heat flux, point C, is called the burnout point or the Lindenfrost point. Although
it is desirable to operate the vapor generators at heat fluxes close to q c , to permit the max-
imum use of the surface area, in most engineering applications it is necessary to control the
heat flux and great care is taken to avoid reaching this point. The primary reason for this is
that, as illustrated, when the heat flux is increased gradually, the temperature rises steadily
until point C is reached. Any increase of heat flux beyond the value of q c , however, will
dramatically change the surface temperature to T T T , typically exceeding the solid
s
e,E
sat
melting point and leading to failure of the material in which the liquid is held or from which
the heater is fabricated.
Nucleate Pool Boiling
The heat flux data are best correlated by 26
q h g( ) c T e 3
1/2
p,l
v
l
lfg
g Ch Pr 1.7
c fg l
where the subscripts l and v denote saturated liquid and vapor, respectively. The surface
tension of the liquid is (N/m). The quantity g is the proportionality constant equal to 1
c
2
2
kg m/N s . The quantity g is the local gravitational acceleration in m/sec . The values of
C are given in Table 23. The above equation may be applied to different geometries, such
as plates, wire, or cylinders.
Table 23 Values of the Constant C for Various Liquid–Surface Combinations
Fluid–Heating Surface Combinations C
Water with polished copper, platinum, or mechanically polished stainless steel 0.0130
Water with brass or nickel 0.006
Water with ground and polished stainless steel 0.008
Water with Teflon-plated stainless steel 0.008