Page 206 - Mechanical Engineers' Handbook (Volume 4)
P. 206
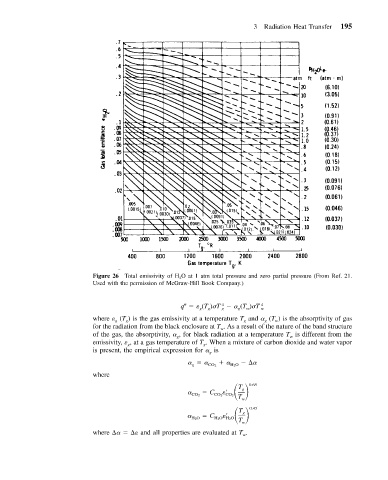
3 Radiation Heat Transfer 195
Figure 26 Total emissivity of H 2 O at 1 atm total pressure and zero partial pressure (From Ref. 21.
Used with the permission of McGraw-Hill Book Company.)
4
q (T ) T (T ) T 4 w
g
w
g
g
g
where (T ) is the gas emissivity at a temperature T and (T ) is the absorptivity of gas
w
g
g
g
g
for the radiation from the black enclosure at T . As a result of the nature of the band structure
w
of the gas, the absorptivity, , for black radiation at a temperature T is different from the
w
g
emissivity, , at a gas temperature of T . When a mixture of carbon dioxide and water vapor
g
g
is present, the empirical expression for is
g
CO 2 H O
g
2
where
C 0.65
T
g
T w
CO 2 CO 2 CO 2
0.45
T
HO C HO HO g
2
2
T w
2
where and all properties are evaluated at T .
w