Page 209 - Mechanical Engineers' Handbook (Volume 4)
P. 209
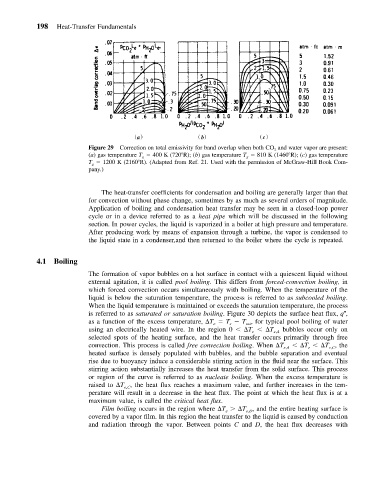
198 Heat-Transfer Fundamentals
Figure 29 Correction on total emissivity for band overlap when both CO 2 and water vapor are present:
(a) gas temperature T g 400 K (720 R); (b) gas temperature T g 810 K (1460 R); (c) gas temperature
T g 1200 K (2160 R). (Adapted from Ref. 21. Used with the permission of McGraw-Hill Book Com-
pany.)
The heat-transfer coefficients for condensation and boiling are generally larger than that
for convection without phase change, sometimes by as much as several orders of magnitude.
Application of boiling and condensation heat transfer may be seen in a closed-loop power
cycle or in a device referred to as a heat pipe which will be discussed in the following
section. In power cycles, the liquid is vaporized in a boiler at high pressure and temperature.
After producing work by means of expansion through a turbine, the vapor is condensed to
the liquid state in a condenser,and then returned to the boiler where the cycle is repeated.
4.1 Boiling
The formation of vapor bubbles on a hot surface in contact with a quiescent liquid without
external agitation, it is called pool boiling. This differs from forced-convection boiling, in
which forced convection occurs simultaneously with boiling. When the temperature of the
liquid is below the saturation temperature, the process is referred to as subcooled boiling.
When the liquid temperature is maintained or exceeds the saturation temperature, the process
is referred to as saturated or saturation boiling. Figure 30 depicts the surface heat flux, q ,
as a function of the excess temperature, T T T , for typical pool boiling of water
s
e
sat
using an electrically heated wire. In the region 0 T T e,A bubbles occur only on
e
selected spots of the heating surface, and the heat transfer occurs primarily through free
convection. This process is called free convection boiling. When T e,A T T , the
e
e,C
heated surface is densely populated with bubbles, and the bubble separation and eventual
rise due to buoyancy induce a considerable stirring action in the fluid near the surface. This
stirring action substantially increases the heat transfer from the solid surface. This process
or region of the curve is referred to as nucleate boiling. When the excess temperature is
raised to T , the heat flux reaches a maximum value, and further increases in the tem-
e,C
perature will result in a decrease in the heat flux. The point at which the heat flux is at a
maximum value, is called the critical heat flux.
Film boiling occurs in the region where T T , and the entire heating surface is
e,D
e
covered by a vapor film. In this region the heat transfer to the liquid is caused by conduction
and radiation through the vapor. Between points C and D, the heat flux decreases with