Page 106 - Membranes for Industrial Wastewater Recovery and Re-Use
P. 106
86 iMPmbranes for Industrial Wastewater Recovery and Re-use
0[ 1
Jan-01 Mar-01 May-01 Jul-01 Sep-01 Nov-01
Date
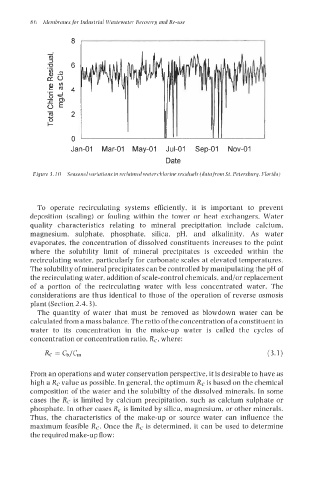
Figure 3. IO Seasonal variations in reclaimed water chlorine residuals (data from St. Petersburg, Florida)
To operate recirculating systems efficiently, it is important to prevent
deposition (scaling) or fouling within the tower or heat exchangers. Water
quality characteristics relating to mineral precipitation include calcium,
magnesium, sulphate, phosphate, silica, pH, and alkalinity. As water
evaporates, the concentration of dissolved constituents increases to the point
where the solubility limit of mineral precipitates is exceeded within the
recirculating water, particularly for carbonate scales at elevated temperatures.
The solubility of mineral precipitates can be controlled by manipulating the pH of
the recirculating water, addition of scale-control chemicals, and/or replacement
of a portion of the recirculating water with less concentrated water. The
considerations are thus identical to those of the operation of reverse osmosis
plant (Section 2.4.3).
The quantity of water that must be removed as blowdown water can be
calculated from a mass balance. The ratio of the concentration of a constituent in
water to its concentration in the make-up water is called the cycles of
concentration or concentration ratio, Rc, where:
RC = cb/cm (3.1)
From an operations and water conservation perspective, it is desirable to have as
high a Rc value as possible. In general, the optimum Rc is based on the chemical
composition of the water and the solubility of the dissolved minerals. In some
cases the Rc is limited by calcium precipitation, such as calcium sulphate or
phosphate. In other cases Rc is limited by silica, magnesium, or other minerals.
Thus, the characteristics of the make-up or source water can influence the
maximum feasible Rc. Once the Rc is determined, it can be used to determine
the required make-up flow: