Page 148 - Modeling of Chemical Kinetics and Reactor Design
P. 148
118 Modeling of Chemical Kinetics and Reactor Design
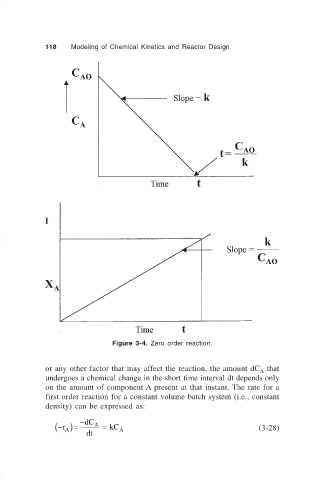
Figure 3-4. Zero order reaction.
or any other factor that may affect the reaction, the amount dC that
A
undergoes a chemical change in the short time interval dt depends only
on the amount of component A present at that instant. The rate for a
first order reaction for a constant volume batch system (i.e., constant
density) can be expressed as:
− ( r A )= −dC A = kC A (3-28)
dt