Page 153 - Modeling of Chemical Kinetics and Reactor Design
P. 153
Reaction Rate Expression 123
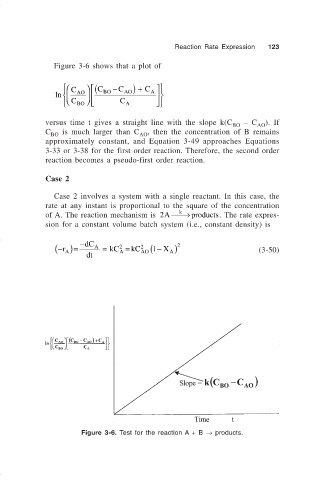
Figure 3-6 shows that a plot of
C C ( BO − C ) + C
A
AO
ln AO
C BO C A
versus time t gives a straight line with the slope k(C BO – C AO ). If
C BO is much larger than C AO , then the concentration of B remains
approximately constant, and Equation 3-49 approaches Equations
3-33 or 3-38 for the first order reaction. Therefore, the second order
reaction becomes a pseudo-first order reaction.
Case 2
Case 2 involves a system with a single reactant. In this case, the
rate at any instant is proportional to the square of the concentration
k
of A. The reaction mechanism is 2A → products. The rate expres-
sion for a constant volume batch system (i.e., constant density) is
− ( r A )= −dC A = kC 2 A = kC 2 AO (1 − X A ) 2 (3-50)
dt
Figure 3-6. Test for the reaction A + B → products.