Page 154 - Modeling of Chemical Kinetics and Reactor Design
P. 154
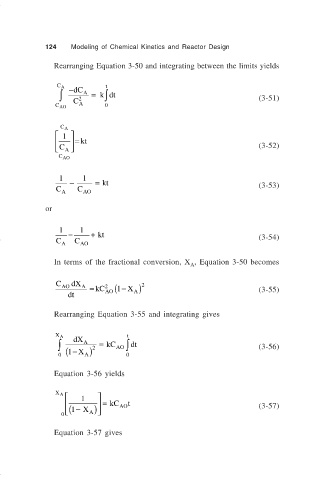
124 Modeling of Chemical Kinetics and Reactor Design
Rearranging Equation 3-50 and integrating between the limits yields
C A −dC t
∫ 2 A = kdt (3-51)
∫
C AO C A 0
C A
1
= kt
C A (3-52)
C AO
1 1
− = kt
C A C AO (3-53)
or
1 1
= + kt
C A C AO (3-54)
In terms of the fractional conversion, X , Equation 3-50 becomes
A
C AO dX A = kC (1 − X ) 2
2
dt AO A (3-55)
Rearranging Equation 3-55 and integrating gives
X A dX t
∫ A 2 = kC AO ∫ dt (3-56)
A
0 ( 1− X ) 0
Equation 3-56 yields
1
X A
( = kC AO t (3-57)
0 1 X− A )
Equation 3-57 gives