Page 158 - Modeling of Chemical Kinetics and Reactor Design
P. 158
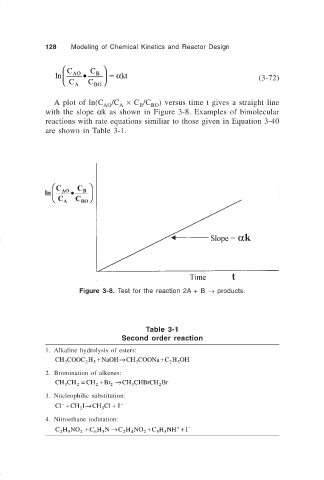
128 Modeling of Chemical Kinetics and Reactor Design
C C
ln AO • B =α kt (3-72)
C A C BO
A plot of ln(C /C × C /C ) versus time t gives a straight line
AO A B BO
with the slope αk as shown in Figure 3-8. Examples of bimolecular
reactions with rate equations similiar to those given in Equation 3-40
are shown in Table 3-1.
Figure 3-8. Test for the reaction 2A + B → products.
Table 3-1
Second order reaction
1. Alkaline hydrolysis of esters:
+
CH COOC H + NaOH→ CH COONa C H OH
3 2 5 3 2 5
2. Bromination of alkenes:
CH CH = CH + Br → CH CHBrCH Br
2
2
2
3
3
2
3. Nucleophilic substitution:
−
Cl + CH I→ CH Cl + I −
3
3
4. Nitroethane iodination:
+
CH NO + C H N → C H NO + C H NH + I −
2
5
5
2
5
2
6
4
2
5