Page 163 - Modeling of Chemical Kinetics and Reactor Design
P. 163
Reaction Rate Expression 133
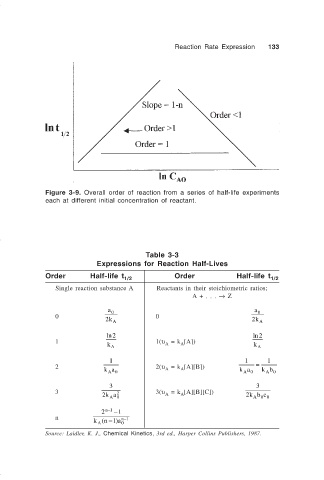
Figure 3-9. Overall order of reaction from a series of half-life experiments
each at different initial concentration of reactant.
Table 3-3
Expressions for Reaction Half-Lives
Order Half-life t Order Half-life t
1/2 1/2
Single reaction substance A Reactants in their stoichiometric ratios;
A + . . . → Z
a 0 a 0
0 0
2 k 2 k
A A
ln2 ln2
1 1(υ = k [A])
k A A A k A
1 1 = 1
2 2(υ = k [A][B])
ka 0 A A ka 0 k b 0
A
A
A
3 3
3 2 3(υ = k [A][B][C])
2ka 0 A A 2kb c
00
A
A
2 n− 1 − 1
n n− 1
k ( n − 1 a ) 0
A
Source: Laidler, K. J., Chemical Kinetics, 3rd ed., Harper Collins Publishers, 1987.