Page 165 - Modeling of Chemical Kinetics and Reactor Design
P. 165
Reaction Rate Expression 135
r dC k
B = B = 1
r C dC C k 2 (3-101)
Integrating Equation 3-101 between the limits yields
C − C k
B BO = 1
C − C CO k 2 (3-102)
C
C − C = k 1 C − k 1 C
or B BO C CO (3-103)
k k
2 2
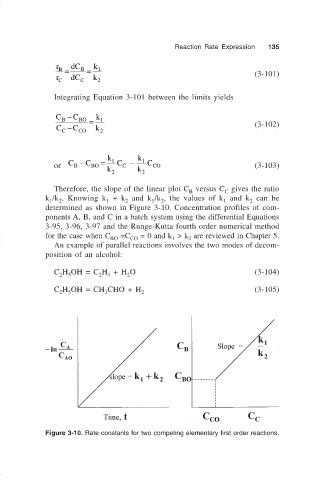
Therefore, the slope of the linear plot C versus C gives the ratio
C
B
k /k . Knowing k + k and k /k , the values of k and k can be
1 2 1 2 1 2 1 2
determined as shown in Figure 3-10. Concentration profiles of com-
ponents A, B, and C in a batch system using the differential Equations
3-95, 3-96, 3-97 and the Runge-Kutta fourth order numerical method
for the case when C =C = 0 and k > k are reviewed in Chapter 5.
CO
BO
1
2
An example of parallel reactions involves the two modes of decom-
position of an alcohol:
C H OH = C H + H O (3-104)
2
5
2
2
4
C H OH = CH CHO + H 2 (3-105)
2
5
3
Figure 3-10. Rate constants for two competing elementary first order reactions.