Page 161 - Modeling of Chemical Kinetics and Reactor Design
P. 161
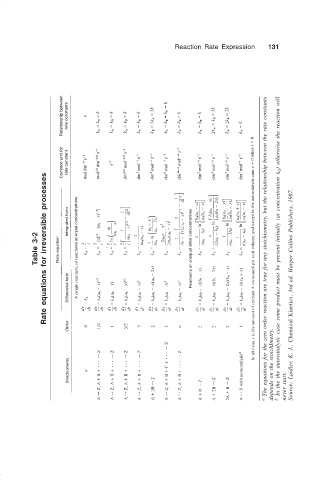
Reaction Rate Expression 131
Table 3-2 Rate equations for irreversible processes a The equations for the zero order reaction are true for any stoichiometry, but the relationship between the rate constants b In the the autocatalytic case some product must be present initially (at concentration x 0 ) otherwise the reaction will Source: Laidler, K. J., Chemical Kinetics, 3rd ed. Harper Collins Publishers, 1987.
depends on the stoichImetry. never start.