Page 156 - Modeling of Chemical Kinetics and Reactor Design
P. 156
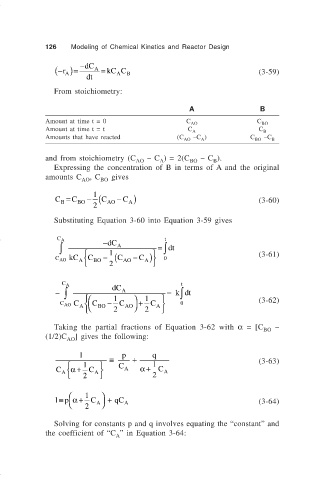
126 Modeling of Chemical Kinetics and Reactor Design
− ( r A )= −dC A = kC C B (3-59)
A
dt
From stoichiometry:
A B
Amount at time t = 0 C AO C BO
Amount at time t = t C C
A B
Amounts that have reacted (C AO –C ) C BO –C B
A
and from stoichiometry (C – C ) = 2(C – C ).
AO A BO B
Expressing the concentration of B in terms of A and the original
amounts C , C gives
AO BO
C = C BO − 1 C ( AO − C ) (3-60)
B
A
2
Substituting Equation 3-60 into Equation 3-59 gives
C A −dC t
∫
∫ A = dt
1
kC − (C − C ) 0 (3-61)
C
C AO A BO AO A
2
C A t
∫
− ∫ dC A = kdt
1
1
C − C + C 0 (3-62)
C AO A C BO AO A
2 2
Taking the partial fractions of Equation 3-62 with α = [C BO –
(1/2)C AO ] gives the following:
1 ≡ p + q
1 C 1 (3-63)
C α + C A α + C A
2 2
A
A
1
1≡ p α + C + qC (3-64)
2 A A
Solving for constants p and q involves equating the “constant” and
the coefficient of “C ” in Equation 3-64:
A