Page 170 - Modeling of Chemical Kinetics and Reactor Design
P. 170
140 Modeling of Chemical Kinetics and Reactor Design
θ + X
ln B A = C AO( θ + ) 1 kt =( C AO + C BO) kt (3-126)
B
θ (1 − X A )
B
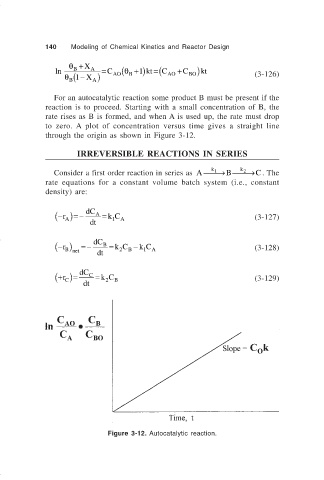
For an autocatalytic reaction some product B must be present if the
reaction is to proceed. Starting with a small concentration of B, the
rate rises as B is formed, and when A is used up, the rate must drop
to zero. A plot of concentration versus time gives a straight line
through the origin as shown in Figure 3-12.
IRREVERSIBLE REACTIONS IN SERIES
Consider a first order reaction in series as A → B → . The
k 2
k 1
C
rate equations for a constant volume batch system (i.e., constant
density) are:
− ( r A )=− dC A = kC A (3-127)
1
dt
− ( r ) =− dC B = kC − k C (3-128)
B net 2 B 1 A
dt
+ ( r C )= dC C = kC B (3-129)
2
dt
Figure 3-12. Autocatalytic reaction.