Page 171 - Modeling of Chemical Kinetics and Reactor Design
P. 171
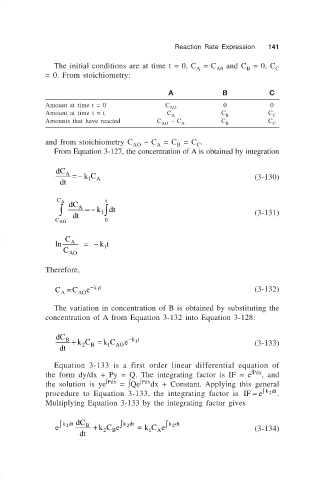
Reaction Rate Expression 141
The initial conditions are at time t = 0, C = C and C = 0, C C
A0
B
A
= 0. From stoichiometry:
A B C
Amount at time t = 0 C 0 0
AO
Amount at time t = t C C C
A B C
Amounts that have reacted C – C C C
AO A B C
and from stoichiometry C AO – C = C = C .
B
A
C
From Equation 3-127, the concentration of A is obtained by integration
dC A =− kC
dt 1 A (3-130)
C A dC t
∫ A =− k 1 ∫ dt
dt (3-131)
C AO 0
C
ln A =− kt
1
C
AO
Therefore,
C = C e − kt 1 (3-132)
A AO
The variation in concentration of B is obtained by substituting the
concentration of A from Equation 3-132 into Equation 3-128:
dC B + kC = k C e − kt 1
dt 2 B 1 AO (3-133)
Equation 3-133 is a first order linear differential equation of
the form dy/dx + Py = Q. The integrating factor is IF = e ∫Pdx , and
the solution is ye ∫Pdx = ∫Qe ∫Pdx dx + Constant. Applying this general
procedure to Equation 3-133, the integrating factor is IF = e ∫ 2 .
kdt
Multiplying Equation 3-133 by the integrating factor gives
∫ kdt dC B ∫ kdt ∫ kdt
2
2
2
e + kC e = k C e (3-134)
1
B
A
2
dt