Page 167 - Modeling of Chemical Kinetics and Reactor Design
P. 167
Reaction Rate Expression 137
improved by incorporating a method that favors the required reaction
over the unwanted reaction.
HOMOGENEOUS CATALYZED REACTIONS
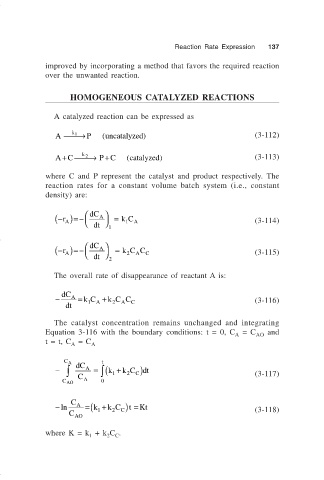
A catalyzed reaction can be expressed as
k 1
P
A → (uncatalyzed) (3-112)
AC → PC (catalyzed) (3-113)
+
+
k 2
where C and P represent the catalyst and product respectively. The
reaction rates for a constant volume batch system (i.e., constant
density) are:
− ( r )=− dC A = kC
A 1 A (3-114)
dt
1
− ( r )=− dC A = kC C (3-115)
A
dt 2 A C
2
The overall rate of disappearance of reactant A is:
− dC A = kC A + k C C C (3-116)
A
2
1
dt
The catalyst concentration remains unchanged and integrating
Equation 3-116 with the boundary conditions: t = 0, C = C AO and
A
t = t, C = C A
A
C A dC t
∫
− ∫ A = (k 1 + k C C )dt
2
C A (3-117)
C AO 0
C
−ln A =(k 1 + k C C ) =t Kt (3-118)
2
C AO
where K = k + k C .
1 2 C