Page 176 - Modeling of Chemical Kinetics and Reactor Design
P. 176
146 Modeling of Chemical Kinetics and Reactor Design
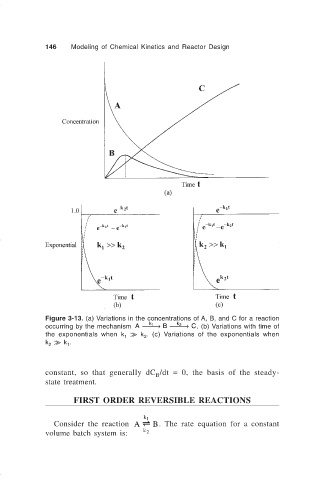
Figure 3-13. (a) Variations in the concentrations of A, B, and C for a reaction
occurring by the mechanism A → B → C. (b) Variations with time of
k 2
k 1
the exponentials when k k k . (c) Variations of the exponentials when
1 2
k k k .
2 1
constant, so that generally dC /dt = 0, the basis of the steady-
B
state treatment.
FIRST ORDER REVERSIBLE REACTIONS
Consider the reaction A [ B. The rate equation for a constant
k 1
volume batch system is: k 2