Page 179 - Modeling of Chemical Kinetics and Reactor Design
P. 179
Reaction Rate Expression 149
At equilibrium with concentrations of A and B as C , C , respectively,
Ae
Be
then k C = k C . Since dC /dt = 0 at equilibrium
2 Be
1 Ae
A
k 1 = K = C Be
k C (3-168)
2 Ae
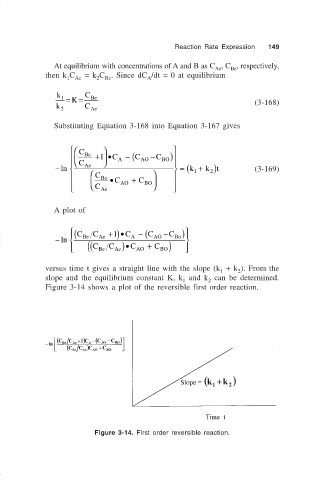
Substituting Equation 3-168 into Equation 3-167 gives
C Be
+1 •C A − (C AO −C BO )
= (k
−ln C Ae 1 + k 2 )t (3-169)
C Be •C + C
Ae AO BO
C
A plot of
( C C + )•C − (C −C )
1
−ln Be Ae A AO ) Bo
( ( C Be C Ae )•C AO + C BO
versus time t gives a straight line with the slope (k + k ). From the
1
2
slope and the equilibrium constant K, k and k can be determined.
1
2
Figure 3-14 shows a plot of the reversible first order reaction.
Figure 3-14. First order reversible reaction.