Page 181 - Modeling of Chemical Kinetics and Reactor Design
P. 181
Reaction Rate Expression 151
All other reversible second-order rate equations have the same
solution with the boundary conditions assumed in Equation 3-176.
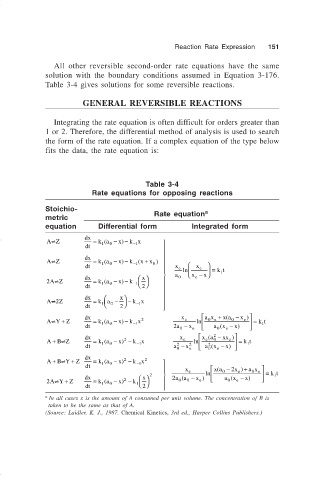
Table 3-4 gives solutions for some reversible reactions.
GENERAL REVERSIBLE REACTIONS
Integrating the rate equation is often difficult for orders greater than
1 or 2. Therefore, the differential method of analysis is used to search
the form of the rate equation. If a complex equation of the type below
fits the data, the rate equation is:
Table 3-4
Rate equations for opposing reactions
Stoichio-
Rate equation a
metric
equation Differential form Integrated form
x −
[
AZ dx = ka − ) k x
(
−1
1
0
dt
x −
+
[
(
AZ dx = ka − ) k ( x x )
0
−1
1
0
dt x e ln x e = kt
1
x
x
e
x −
[
2 AZ dx = ka − ) k −1 a 0 x −
(
1
0
dt 2
dx x
A[2 Z = ka − − kx
1
0
−1
dt 2
x a −
[ +
x −
e
0 e
AY Z dx = ka − ) k x 2 x e ln ax + ( 0 x ) = kt
(
0
−1
1
1
(
dt 2 2a − x e ax − x)
e
0
0
2
(
0
+ [
e
e
AB Z dx = ka − x) 2 − k x x e ln xa − xx ) = kt
(
−
1
1
0
1
2
2
dt a − x 2 ax − x)
(
0 e 0 e
+ [
+
2
)
(
AB Y Z dx = ka − x) − kx 2
−
0
1
1
dt x e ln xa ( 0 − 2 x ) + a x = kt
0
e
e
x
[
+
0
e
0
2
2AY Z dx = ka 0 − x ) − k 1 2 2 aa ( 0 − x ) ax ( e − x) 1
(
1
dt 2
a
In all cases x is the amount of A consumed per unit volume. The concentration of B is
taken to be the same as that of A.
(Source: Laidler, K. J., 1987. Chemical Kinetics, 3rd ed., Harper Collins Publishers.)