Page 177 - Modeling of Chemical Kinetics and Reactor Design
P. 177
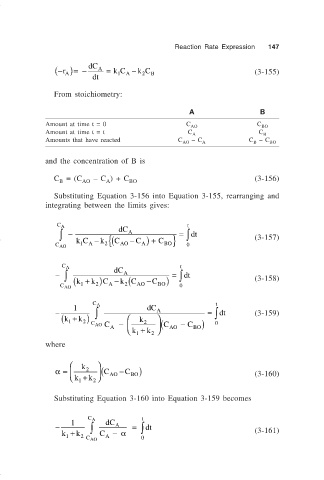
Reaction Rate Expression 147
− ( r A )=− dC A = kC A − k C B (3-155)
1
2
dt
From stoichiometry:
A B
Amount at time t = 0 C AO C BO
Amount at time t = t C A C B
Amounts that have reacted C – C C – C
AO A B BO
and the concentration of B is
C = (C – C ) + C (3-156)
B AO A BO
Substituting Equation 3-156 into Equation 3-155, rearranging and
integrating between the limits gives:
C A dC t
∫ − − { A = dt (3-157)
∫
kC A k 2 (C AO − C A ) + C BO }
1
C AO 0
C A t
∫
− ∫ dC A = dt
k
(k 1 + )C A − (C AO −C BO ) (3-158)
k
2
2
C AO 0
C A dC t
∫
− 1 ∫ A = dt (3-159)
k
k + ) k
( 1 2 2 ) 0
C AO C − (C − C
A AO BO
k 1 + k 2
where
k
α= 2 (C AO −C BO ) (3-160)
k 1 + k 2
Substituting Equation 3-160 into Equation 3-159 becomes
C A t
− 1 ∫ dC A = ∫ dt
k 1 + k 2 C A − α (3-161)
C AO 0