Page 150 - Modeling of Chemical Kinetics and Reactor Design
P. 150
120 Modeling of Chemical Kinetics and Reactor Design
Rearranging and integrating Equation 3-37 between the limits t =
0, X = 0 and t = t and X = X gives
A
f
A
Af
X Af dX t f
∫
∫ ( X ) = kdt
A
0 1− A 0
−
− (1 X Af ) = kt f (3-38)
ln
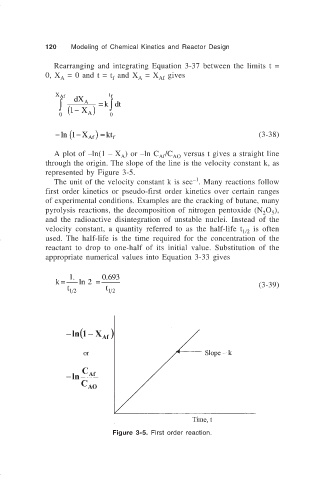
A plot of –ln(1 – X ) or –ln C /C AO versus t gives a straight line
A
Af
through the origin. The slope of the line is the velocity constant k, as
represented by Figure 3-5.
–1
The unit of the velocity constant k is sec . Many reactions follow
first order kinetics or pseudo-first order kinetics over certain ranges
of experimental conditions. Examples are the cracking of butane, many
pyrolysis reactions, the decomposition of nitrogen pentoxide (N O ),
2
5
and the radioactive disintegration of unstable nuclei. Instead of the
velocity constant, a quantity referred to as the half-life t is often
1/2
used. The half-life is the time required for the concentration of the
reactant to drop to one-half of its initial value. Substitution of the
appropriate numerical values into Equation 3-33 gives
.
k = 1. ln 2 = 0 693
t 12 t 12 (3-39)
Figure 3-5. First order reaction.