Page 21 - Modeling of Chemical Kinetics and Reactor Design
P. 21
the tank from refrigerated storage will expand to cause the tank to be
full of liquid as it heats. The tank vent would then open and subsequently
leak liquid ammonia to the atmosphere. Training personnel for the
hazardous nature of anhydrous ammonia and how to handle emergency
conditions in the plant is essential.
The American Conference of Government Industrial Hygienists
(ACGIH) has established threshold doses called the threshold limit
values (TLVs) for many chemical agents. The TLV is the airborne
concentration that corresponds to conditions where no adverse effects
are normally expected during a worker’s lifetime. The exposure is
assumed to occur only during normal working hours, eight hours per
day and five days per week.
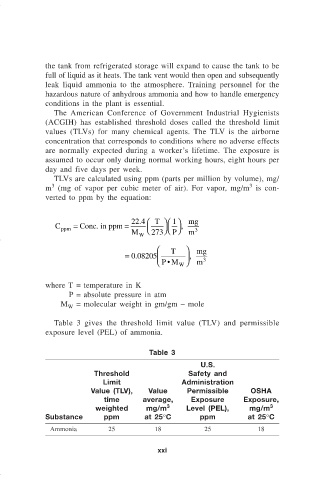
TLVs are calculated using ppm (parts per million by volume), mg/
3
3
m (mg of vapor per cubic meter of air). For vapor, mg/m is con-
verted to ppm by the equation:
1
C = Conc. in ppm = 22.4 T , mg
ppm 3
M W 273 P m
T mg
= . 0 08205 ,
PM
• W m 3
where T = temperature in K
P = absolute pressure in atm
M = molecular weight in gm/gm – mole
W
Table 3 gives the threshold limit value (TLV) and permissible
exposure level (PEL) of ammonia.
Table 3
U.S.
Threshold Safety and
Limit Administration
Value (TLV), Value Permissible OSHA
time average, Exposure Exposure,
weighted mg/m 3 Level (PEL), mg/m 3
Substance ppm at 25°C ppm at 25°C
Ammonia 25 18 25 18
xxi