Page 223 - Modeling of Chemical Kinetics and Reactor Design
P. 223
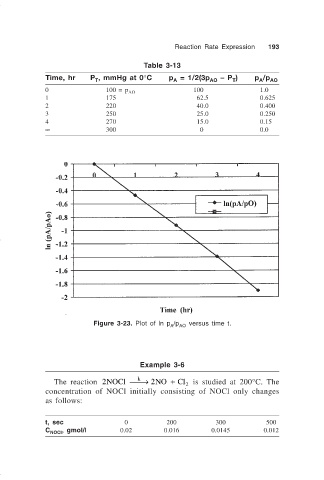
Reaction Rate Expression 193
Table 3-13
Time, hr P , mmHg at 0°C p = 1/2(3p AO – P ) p /p AO
T
A
T
A
0 100 = p 100 1.0
AO
1 175 62.5 0.625
2 220 40.0 0.400
3 250 25.0 0.250
4 270 15.0 0.15
∞ 300 0 0.0
Figure 3-23. Plot of ln p /p versus time t.
A AO
Example 3-6
The reaction 2NOCl → 2NO + Cl is studied at 200°C. The
k
2
concentration of NOCl initially consisting of NOCl only changes
as follows:
t, sec 0 200 300 500
C NOCl , gmol/l 0.02 0.016 0.0145 0.012