Page 225 - Modeling of Chemical Kinetics and Reactor Design
P. 225
Reaction Rate Expression 195
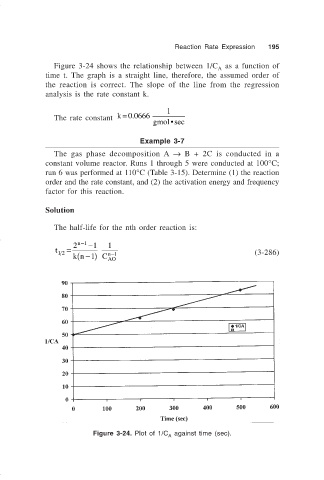
Figure 3-24 shows the relationship between 1/C as a function of
A
time t. The graph is a straight line, therefore, the assumed order of
the reaction is correct. The slope of the line from the regression
analysis is the rate constant k.
l
The rate constant k =0 0666.
gmol •sec
Example 3-7
The gas phase decomposition A → B + 2C is conducted in a
constant volume reactor. Runs 1 through 5 were conducted at 100°C;
run 6 was performed at 110°C (Table 3-15). Determine (1) the reaction
order and the rate constant, and (2) the activation energy and frequency
factor for this reaction.
Solution
The half-life for the nth order reaction is:
−
−
n 1
1
2
t 12 = kn 1− ) C 1 − (3-286)
(
n 1
AO
Figure 3-24. Plot of 1/C against time (sec).
A