Page 220 - Modeling of Chemical Kinetics and Reactor Design
P. 220
190 Modeling of Chemical Kinetics and Reactor Design
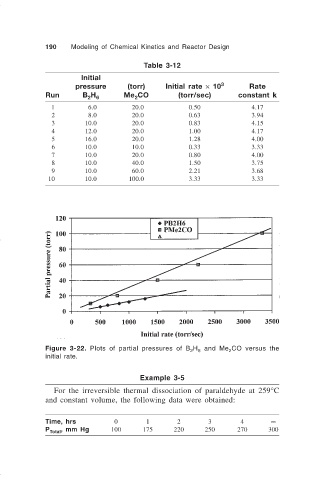
Table 3-12
Initial
pressure (torr) Initial rate × 10 3 Rate
Run B H 6 Me CO (torr/sec) constant k
2
2
11 6.0 20.0 0.50 4.17
12 8.0 20.0 0.63 3.94
13 10.0 20.0 0.83 4.15
14 12.0 20.0 1.00 4.17
15 16.0 20.0 1.28 4.00
16 10.0 10.0 0.33 3.33
17 10.0 20.0 0.80 4.00
18 10.0 40.0 1.50 3.75
19 10.0 60.0 2.21 3.68
10 10.0 100.0 3.33 3.33
Figure 3-22. Plots of partial pressures of B H and Me CO versus the
2
6
2
initial rate.
Example 3-5
For the irreversible thermal dissociation of paraldehyde at 259°C
and constant volume, the following data were obtained:
Time, hrs 0 1 2 3 4 ∞
P Total , mm Hg 100 175 220 250 270 300