Page 218 - Modeling of Chemical Kinetics and Reactor Design
P. 218
188 Modeling of Chemical Kinetics and Reactor Design
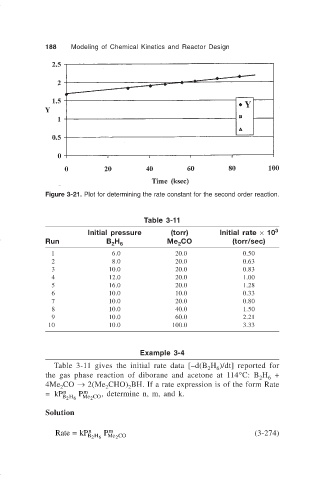
Figure 3-21. Plot for determining the rate constant for the second order reaction.
Table 3-11
Initial pressure (torr) Initial rate × 10 3
Run B H Me CO (torr/sec)
2 6 2
11 6.0 20.0 0.50
12 8.0 20.0 0.63
13 10.0 20.0 0.83
14 12.0 20.0 1.00
15 16.0 20.0 1.28
16 10.0 10.0 0.33
17 10.0 20.0 0.80
18 10.0 40.0 1.50
19 10.0 60.0 2.21
10 10.0 100.0 3.33
Example 3-4
Table 3-11 gives the initial rate data [–d(B H )/dt] reported for
6
2
the gas phase reaction of diborane and acetone at 114°C: B H +
2
6
4Me CO → 2(Me CHO) BH. If a rate expression is of the form Rate
2
2
2
= kP n P m , determine n, m, and k.
2
2
BH 6 Me CO
Solution
Rate = kP n P m (3-274)
2
BH 6 Me CO
2