Page 269 - Modeling of Chemical Kinetics and Reactor Design
P. 269
Industrial and Laboratory Reactors 239
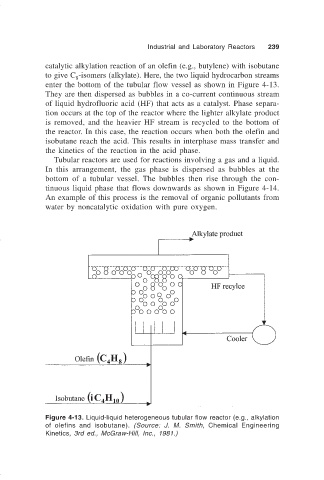
catalytic alkylation reaction of an olefin (e.g., butylene) with isobutane
to give C -isomers (alkylate). Here, the two liquid hydrocarbon streams
8
enter the bottom of the tubular flow vessel as shown in Figure 4-13.
They are then dispersed as bubbles in a co-current continuous stream
of liquid hydrofluoric acid (HF) that acts as a catalyst. Phase separa-
tion occurs at the top of the reactor where the lighter alkylate product
is removed, and the heavier HF stream is recycled to the bottom of
the reactor. In this case, the reaction occurs when both the olefin and
isobutane reach the acid. This results in interphase mass transfer and
the kinetics of the reaction in the acid phase.
Tubular reactors are used for reactions involving a gas and a liquid.
In this arrangement, the gas phase is dispersed as bubbles at the
bottom of a tubular vessel. The bubbles then rise through the con-
tinuous liquid phase that flows downwards as shown in Figure 4-14.
An example of this process is the removal of organic pollutants from
water by noncatalytic oxidation with pure oxygen.
Figure 4-13. Liquid-liquid heterogeneous tubular flow reactor (e.g., alkylation
of olefins and isobutane). (Source: J. M. Smith, Chemical Engineering
Kinetics, 3rd ed., McGraw-Hill, Inc., 1981.)