Page 239 - Modern Analytical Chemistry
P. 239
1400-CH07 9/8/99 4:04 PM Page 222
222 Modern Analytical Chemistry
EXAMPLE .17
7
2+
A divalent metal ion, M , is to be extracted from an aqueous solution into an
organic solvent using a chelating agent, HL, dissolved in the organic solvent.
The partition coefficients for the chelating agent, K D,L , and the metal–ligand
4
4
complex, K D,c , are 1.0 ´10 and 7.0 ´10 , respectively. The acid dissociation
–5
constant for the chelating agent, K a , is 5.0 ´10 , and the formation constant
16
for the metal–ligand complex, b, is 2.5 ´10 . Calculate the extraction
–6
2+
efficiency when 100.0 mL of a 1.0 ´10 M aqueous solution of M , buffered
to a pH of 1.00, is extracted with 10.00 mL of an organic solvent that is 0.1 mM
in the chelating agent. Repeat the calculation at a pH of 3.00.
SOLUTION
+
At a pH of 1.00 ([H 3 O aq ] = 0.10), the distribution ratio for the extraction is
)
(. 25 ´10 16 )( . 70 ´ 10 4 )(. 50 ´ 10 -5 2 ´ 10 -4 2
) ( . 10
D = 42 2 16 -5 2 -4 2 = . 0 0438
0 10
(. 1 0 ´10 ) ( . ) +( . 2 5 ´ 10 )( . 5 0 ´ 10 ) (. 1 0 ´ 10 )
and the fraction of metal ion remaining in the aqueous phase is
0
100 . mL
)
(Q aq 1 = = . 0 996
(0.0438)(10.00 mL ) + 100 . mL
0
Thus, at a pH of 1.00, only 0.40% of the metal is extracted. Changing the pH to
3.00, however, gives an extraction efficiency of 97.8%. A plot of extraction
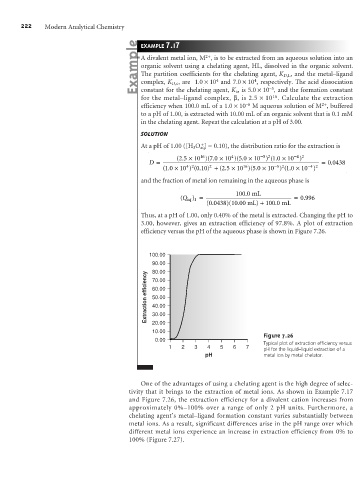
efficiency versus the pH of the aqueous phase is shown in Figure 7.26.
100.00
90.00
80.00
Extraction efficiency 70.00
60.00
50.00
40.00
30.00
20.00
10.00
Figure 7.26
0.00
Typical plot of extraction efficiency versus
1 2 3 4 5 6 7 pH for the liquid–liquid extraction of a
pH metal ion by metal chelator.
One of the advantages of using a chelating agent is the high degree of selec-
tivity that it brings to the extraction of metal ions. As shown in Example 7.17
and Figure 7.26, the extraction efficiency for a divalent cation increases from
approximately 0%–100% over a range of only 2 pH units. Furthermore, a
chelating agent’s metal–ligand formation constant varies substantially between
metal ions. As a result, significant differences arise in the pH range over which
different metal ions experience an increase in extraction efficiency from 0% to
100% (Figure 7.27).