Page 320 - Modern Analytical Chemistry
P. 320
1400-CH09 9/9/99 2:12 PM Page 303
Chapter 9 Titrimetric Methods of Analysis 303
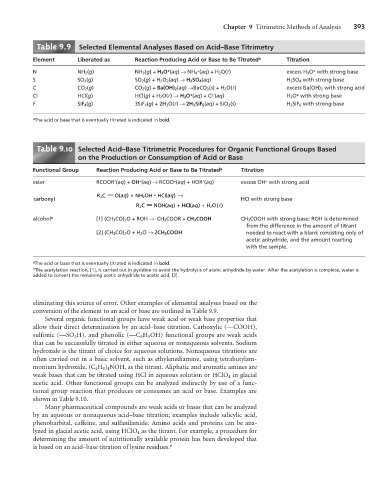
Table 9.9 Selected Elemental Analyses Based on Acid–Base Titrimetry
Element Liberated as Reaction Producing Acid or Base to Be Titrated a Titration
+
+
+
N NH 3 (g) NH 3 (g)+ H 3 O (aq) ® NH 4 (aq)+H 2 O(l) excess H 3 O with strong base
S SO 2 (g) SO 2 (g)+H 2 O 2 (aq) ® H 2 SO 4 (aq) H 2 SO 4 with strong base
C CO 2 (g) CO 2 (g)+ Ba(OH) 2 (aq) ® BaCO 3 (s)+H 2 O(l) excess Ba(OH) 2 with strong acid
+
+
–
Cl HCl(g) HCl(g)+H 2 O(l) ® H 3 O (aq)+Cl (aq) H 3 O with strong base
F SiF 4 (g) 3SiF 4 (g)+2H 2 O(l) ® 2H 2 SiF 6 (aq) + SiO 2 (s) H 2 SiF 6 with strong base
a The acid or base that is eventually titrated is indicated in bold.
Table 9 .10 Selected Acid–Base Titrimetric Procedures for Organic Functional Groups Based
on the Production or Consumption of Acid or Base
Functional Group Reaction Producing Acid or Base to Be Titrated a Titration
–
ester RCOOR’(aq)+ OH (aq) ® RCOO (aq) + HOR’(aq) excess OH with strong acid
–
–
2 ×
—
)
R C — O(aq + NH OH HCI(aq ®
)
2
carbonyl HCI with strong base
—
R C — NOH(aq +HCI (aq + H O( )
l
)
)
2
2
alcohol b [1] (CH 3 CO) 2 O + ROH ® CH 3 COOR + CH 3 COOH CH 3 COOH with strong base; ROH is determined
from the difference in the amount of titrant
[2] (CH 3 CO) 2 O+H 2 O ® 2CH 3 COOH needed to react with a blank consisting only of
acetic anhydride, and the amount reacting
with the sample.
a The acid or base that is eventually titrated is indicated in bold.
b The acetylation reaction, [1], is carried out in pyridine to avoid the hydrolysis of acetic anhydride by water. After the acetylation is complete, water is
added to convert the remaining acetic anhydride to acetic acid, [2].
eliminating this source of error. Other examples of elemental analyses based on the
conversion of the element to an acid or base are outlined in Table 9.9.
Several organic functional groups have weak acid or weak base properties that
allow their direct determination by an acid–base titration. Carboxylic (—COOH),
sulfonic (—SO 3 H), and phenolic (—C 6 H 5 OH) functional groups are weak acids
that can be successfully titrated in either aqueous or nonaqueous solvents. Sodium
hydroxide is the titrant of choice for aqueous solutions. Nonaqueous titrations are
often carried out in a basic solvent, such as ethylenediamine, using tetrabutylam-
monium hydroxide, (C 4 H 9 ) 4 NOH, as the titrant. Aliphatic and aromatic amines are
weak bases that can be titrated using HCl in aqueous solution or HClO 4 in glacial
acetic acid. Other functional groups can be analyzed indirectly by use of a func-
tional group reaction that produces or consumes an acid or base. Examples are
shown in Table 9.10.
Many pharmaceutical compounds are weak acids or bases that can be analyzed
by an aqueous or nonaqueous acid–base titration; examples include salicylic acid,
phenobarbital, caffeine, and sulfanilamide. Amino acids and proteins can be ana-
lyzed in glacial acetic acid, using HClO 4 as the titrant. For example, a procedure for
determining the amount of nutritionally available protein has been developed that
is based on an acid–base titration of lysine residues. 6