Page 318 - Modern Analytical Chemistry
P. 318
1400-CH09 9/9/99 2:12 PM Page 301
Chapter 9 Titrimetric Methods of Analysis 301
14.0
12.0
10.0
pH 8.0
6.0
4.0
2.0
0.0
0.00 10.00 20.00 30.00 40.00 50.00 60.00 70.00
Volume of titrant
(a)
14.0
12.0
10.0
pH 8.0
6.0
4.0
2.0
0.0
0.00 20.00 40.00 60.00 80.00 100.00 120.00 140.00
Volume of titrant
(b)
14.0
12.0
10.0
pH 8.0
6.0
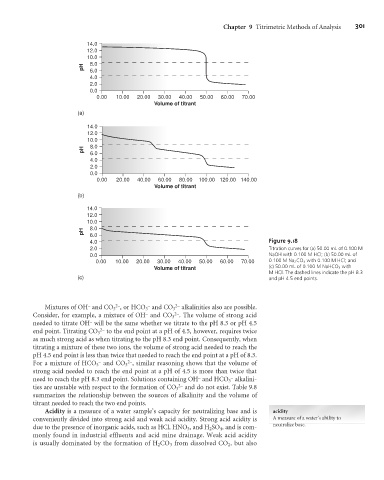
4.0 Figure 9.18
2.0 Titration curves for (a) 50.00 mL of 0.100 M
0.0 NaOH with 0.100 M HCl; (b) 50.00 mL of
0.00 10.00 20.00 30.00 40.00 50.00 60.00 70.00 0.100 M Na 2 CO 3 with 0.100 M HCl; and
Volume of titrant (c) 50.00 mL of 0.100 M NaHCO 3 with
M HCl. The dashed lines indicate the pH 8.3
(c) and pH 4.5 end points.
–
–
2–
2–
Mixtures of OH and CO 3 , or HCO 3 and CO 3 alkalinities also are possible.
–
2–
Consider, for example, a mixture of OH and CO 3 . The volume of strong acid
–
needed to titrate OH will be the same whether we titrate to the pH 8.3 or pH 4.5
2–
end point. Titrating CO 3 to the end point at a pH of 4.5, however, requires twice
as much strong acid as when titrating to the pH 8.3 end point. Consequently, when
titrating a mixture of these two ions, the volume of strong acid needed to reach the
pH 4.5 end point is less than twice that needed to reach the end point at a pH of 8.3.
2–
–
For a mixture of HCO 3 and CO 3 , similar reasoning shows that the volume of
strong acid needed to reach the end point at a pH of 4.5 is more than twice that
–
–
need to reach the pH 8.3 end point. Solutions containing OH and HCO 3 alkalini-
2–
ties are unstable with respect to the formation of CO 3 and do not exist. Table 9.8
summarizes the relationship between the sources of alkalinity and the volume of
titrant needed to reach the two end points.
Acidity is a measure of a water sample’s capacity for neutralizing base and is acidity
conveniently divided into strong acid and weak acid acidity. Strong acid acidity is A measure of a water’s ability to
due to the presence of inorganic acids, such as HCl, HNO 3 , and H 2 SO 4 , and is com- neutralize base.
monly found in industrial effluents and acid mine drainage. Weak acid acidity
is usually dominated by the formation of H 2 CO 3 from dissolved CO 2 , but also