Page 321 - Modern Analytical Chemistry
P. 321
1400-CH09 9/9/99 2:12 PM Page 304
304 Modern Analytical Chemistry
Quantitative Calculations In acid–base titrimetry the quantitative relationship be-
tween the analyte and the titrant is determined by the stoichiometry of the relevant
reactions. As outlined in Section 2C, stoichiometric calculations may be simplified
by focusing on appropriate conservation principles. In an acid–base reaction the
number of protons transferred between the acid and base is conserved; thus
+
+
moles of H donated moles of H accepted
´ moles acid = ´ moles base
mole acid mole base
The following example demonstrates the application of this approach in the direct
analysis of a single analyte.
9
EXAMPLE .2
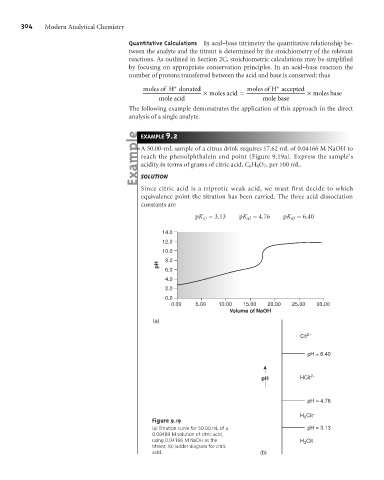
A 50.00-mL sample of a citrus drink requires 17.62 mL of 0.04166 M NaOH to
reach the phenolphthalein end point (Figure 9.19a). Express the sample’s
acidity in terms of grams of citric acid, C 6 H 8 O 7 , per 100 mL.
SOLUTION
Since citric acid is a triprotic weak acid, we must first decide to which
equivalence point the titration has been carried. The three acid dissociation
constants are
pK a1 = 3.13 pK a2 = 4.76 pK a3 = 6.40
14.0
12.0
10.0
8.0
pH
6.0
4.0
2.0
0.0
0.00 5.00 10.00 15.00 20.00 25.00 30.00
Volume of NaOH
(a)
Cit 3–
pH = 6.40
pH HCit 2–
pH = 4.76
H Cit –
2
Figure 9.19
(a) Titration curve for 50.00 mL of a pH = 3.13
0.00489 M solution of citric acid,
using 0.04166 M NaOH as the H Cit
3
titrant; (b) ladder diagram for citric
acid. (b)