Page 316 - Modern Analytical Chemistry
P. 316
1400-CH09 9/9/99 2:12 PM Page 299
Chapter 9 Titrimetric Methods of Analysis 299
9 7
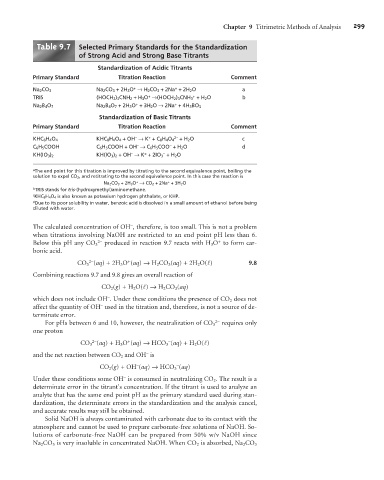
Table . Selected Primary Standards for the Standardization
of Strong Acid and Strong Base Titrants
Standardization of Acidic Titrants
Primary Standard Titration Reaction Comment
+
+
Na 2 CO 3 Na 2 CO 3 +2H 3 O ® H 2 CO 3 + 2Na +2H 2 O a
+
TRIS (HOCH 2 ) 3 CNH 2 +H 3 O ® (HOCH 2 ) 3 CNH 3 +H 2 O b
+
+ +
Na 2 B 4 O 7 Na 2 B 4 O 7 +2H 3 O +3H 2 O ® 2Na +4H 3 BO 3
Standardization of Basic Titrants
Primary Standard Titration Reaction Comment
– + 2– +H 2 O c
KHC 8 H 4 O 4 KHC 8 H 4 O 4 +OH ® K +C 8 H 4 O 4
–
–
C 6 H 5 COOH C 6 H 5 COOH + OH ® C 6 H 5 COO +H 2 O d
+
–
–
KH(IO 3 ) 2 KH(IO 3 ) 2 +OH ® K + 2IO 3 +H 2 O
a The end point for this titration is improved by titrating to the second equivalence point, boiling the
solution to expel CO 2 , and retitrating to the second equivalence point. In this case the reaction is
+
+
Na 2 CO 3 +2H 3 O ® CO 2 + 2Na +3H 2 O
b TRIS stands for tris-(hydroxymethyl)aminomethane.
c KHC 8 H 4 O 4 is also known as potassium hydrogen phthalate, or KHP.
d Due to its poor solubility in water, benzoic acid is dissolved in a small amount of ethanol before being
diluted with water.
–
The calculated concentration of OH , therefore, is too small. This is not a problem
when titrations involving NaOH are restricted to an end point pH less than 6.
2– +
Below this pH any CO 3 produced in reaction 9.7 reacts with H 3 O to form car-
bonic acid.
+
2–
CO 3 (aq)+2H 3 O (aq) ® H 2 CO 3 (aq)+2H 2 O(l) 9.8
Combining reactions 9.7 and 9.8 gives an overall reaction of
CO 2 (g)+H 2 O(l) ® H 2 CO 3 (aq)
–
which does not include OH . Under these conditions the presence of CO 2 does not
–
affect the quantity of OH used in the titration and, therefore, is not a source of de-
terminate error.
2–
For pHs between 6 and 10, however, the neutralization of CO 3 requires only
one proton
–
2–
+
CO 3 (aq)+H 3 O (aq) ® HCO 3 (aq)+H 2 O(l)
–
and the net reaction between CO 2 and OH is
–
–
CO 2 (g)+OH (aq) ® HCO 3 (aq)
–
Under these conditions some OH is consumed in neutralizing CO 2 . The result is a
determinate error in the titrant’s concentration. If the titrant is used to analyze an
analyte that has the same end point pH as the primary standard used during stan-
dardization, the determinate errors in the standardization and the analysis cancel,
and accurate results may still be obtained.
Solid NaOH is always contaminated with carbonate due to its contact with the
atmosphere and cannot be used to prepare carbonate-free solutions of NaOH. So-
lutions of carbonate-free NaOH can be prepared from 50% w/v NaOH since
Na 2 CO 3 is very insoluble in concentrated NaOH. When CO 2 is absorbed, Na 2 CO 3