Page 311 - Modern Analytical Chemistry
P. 311
1400-CH09 9/9/99 2:12 PM Page 294
294 Modern Analytical Chemistry
9
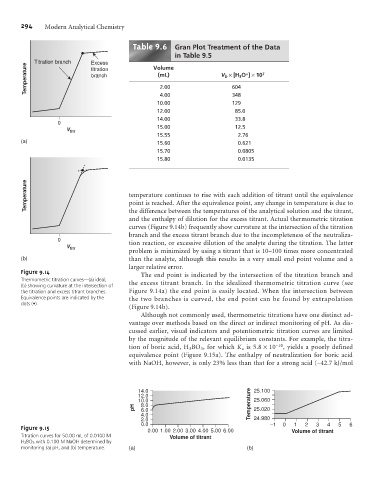
Table .6 Gran Plot Treatment of the Data
in Table 9.5
Titration branch Excess Volume
Temperature branch (mL) V b ´[H 3 O ] ´10 7
titration
+
2.00
604
4.00
10.00 348
129
12.00 85.0
14.00 33.8
0
V titr 15.00 12.5
15.55 2.76
(a) 15.60 0.621
15.70 0.0805
15.80 0.0135
Temperature temperature continues to rise with each addition of titrant until the equivalence
point is reached. After the equivalence point, any change in temperature is due to
the difference between the temperatures of the analytical solution and the titrant,
and the enthalpy of dilution for the excess titrant. Actual thermometric titration
curves (Figure 9.14b) frequently show curvature at the intersection of the titration
branch and the excess titrant branch due to the incompleteness of the neutraliza-
0 tion reaction, or excessive dilution of the analyte during the titration. The latter
V titr
problem is minimized by using a titrant that is 10–100 times more concentrated
(b) than the analyte, although this results in a very small end point volume and a
larger relative error.
Figure 9.14
The end point is indicated by the intersection of the titration branch and
Thermometric titration curves—(a) ideal;
(b) showing curvature at the intersection of the excess titrant branch. In the idealized thermometric titration curve (see
the titration and excess titrant branches. Figure 9.14a) the end point is easily located. When the intersection between
Equivalence points are indicated by the the two branches is curved, the end point can be found by extrapolation
dots (•).
(Figure 9.14b).
Although not commonly used, thermometric titrations have one distinct ad-
vantage over methods based on the direct or indirect monitoring of pH. As dis-
cussed earlier, visual indicators and potentiometric titration curves are limited
by the magnitude of the relevant equilibrium constants. For example, the titra-
tion of boric acid, H 3 BO 3 , for which K a is 5.8 ´10 –10 , yields a poorly defined
equivalence point (Figure 9.15a). The enthalpy of neutralization for boric acid
with NaOH, however, is only 23% less than that for a strong acid (–42.7 kJ/mol
14.0 25.100
12.0
10.0 25.060
pH 8.0 Temperature 25.020
6.0
4.0
2.0 24.980
0.0 –1 0 1 2 3 4 5 6
Figure 9.15 0.00 1.00 2.00 3.00 4.00 5.00 6.00 Volume of titrant
Titration curves for 50.00 mL of 0.0100 M Volume of titrant
H 3 BO 3 with 0.100 M NaOH determined by
monitoring (a) pH, and (b) temperature. (a) (b)